© Springer-Verlag Wien 2015
Hartmut Geiger, Heinrich Jasper and Maria Carolina Florian (eds.)Stem Cell Aging: Mechanisms, Consequences, Rejuvenation10.1007/978-3-7091-1232-8_1616. Closing the Circle: Stem Cell Rejuvenation and Longevity
(1)
Institute for Molecular Medicine, University of Ulm, James Franck-Ring 11c, Ulm, 89081, Germany
(2)
Department of Experimental Hematology and Cancer Biology, Cincinnati Children Hospital Medical Centre (CCHMC), 3333 Burnet Ave, Cincinnati, OH 45229, USA
Abstract
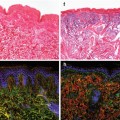
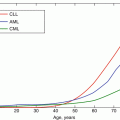
Many organs with high cell turnover (e.g., intestine and blood as well as the germ line) are composed of short-lived cells that require continuous replenishment by stem cells (Potten and Morris J Cell Sci Suppl 10:45–62, 1988; Morrison et al. Annu Rev Cell Dev Biol 11: 35–71, 1995; Fuchs et al. Cell 100(1):143–155, 2000; Tani et al. Proc Natl Acad Sci U S A 97(20):10960–10965, 2000; Stappenbeck et al. Proc Natl Acad Sci U S A 100(3):1004–1009, 2003). Aging results in the inability of these tissues to maintain homeostasis. A number of theories have been proposed regarding the cellular and molecular mechanisms regulating aging, and genetic, behavioral, and environmental factors may all be involved. Declines in the functional capacity of both somatic organ cells but also adult stem cells impair tissue maintenance and regeneration during aging, which may limit lifespan and thus longevity. Tissues that depend on stem cell activity for long-term tissues maintenance of course will be most vulnerable to stem cell aging. High incidences of anemia, impaired wound healing, and intestinal dysfunction in geriatric patients indicate that such alteration can affect the health status of aging humans (de Craen et al. BMJ 327(7407):131–132, 2003). In addition, an age-dependent impairment of stem cell function and a reduction in regenerative capacity can limit stress reactions in response to diseases. Along these lines, “old age” represents a major risk factor for the evolution of, for example, liver cirrhosis as a consequence of chronic hepatitis (Poynard et al. J Hepatol 38(3):257–265, 2003) or various forms of leukemia. Vas et al. PLoS One 7(8):e42080, 2012).
Stem cells are defined by their long-term self-renewal as well as multi-lineage differentiation ability, which a priori should lead to “immortal stem cells.” Consequently, stem cells were initially thought to be endowed with unlimited self-renewal capacity and thus exempt from aging. However, as it has also been reported in this book, a measurable and successive age-dependent decline in stem cell activity from adulthood to old age exists, ranging from yeast to worms to flies to mice and human beings and include prominent stem cell populations like germ line stem cells, hematopoietic, intestinal, skin, retinoid, and muscle stem cells. Aging of stem cell thus (1) exists and (2) clearly contributes to tissue aging and (3) limits by this means healthy aging.
16.1 Stem Cell Rejuvenation: Mechanistic Prerequisites
The identification of mechanisms under which aged stem cells become functionally similar to young stem cells is an important step toward devising clinical treatments of aging-associated imbalance in tissue homeostasis. Attenuating or even reverting stem cell aging might improve tissue regeneration, lead to healthy aging, and might even contribute to longevity. One central prerequisite to achieve that aims though is that the unwanted or causal changes and also the damage that is associated with aging of stem cells are actually reversible or upon their halt or reversion do not further contribute to stem cell aging. While this is obviously a very basic and logical requirement, if translated into biology and mechanisms, things are getting a little more complicated. In general tough, the observation that cellular differentiation is reversible, which was awarded the Nobel Prize in medicine in 2012, proved that stem cell specification and changes in cells over time are in general not associated with irreversible modifications. So, it will be worthwhile to further explore whether, in contrast to what is frequently suggested, aging of stem cells can also be attenuated or reversed.
Mechanisms of aging have been presented in this book so far on a large variety of organisms and types of stem cells. Such mechanisms of stem cell aging include, but are not restricted to, altered cellular communication; genome and telomere instability; epigenetic alterations; changes in proteostasis; altered nutrient sensing; reactive oxygen species as well as mitochondrial dysfunction, primarily due to mutations in the mitochondrial genome; and, especially important to stem cells, aging of the stem cell niche (Rando 2006). They have been referred to as hallmarks of aging (Lopez-Otin et al. 2013). In general, mechanisms that are irreversible like DNA damage, genomic instability, as well as mitochondrial mutations, which might be able to drive premature aging of stem cells (Rossi et al. 2007; Norddahl et al. 2011; Beerman et al. 2014), will not fulfill this critical requirement of reversibility. Reactive oxygen species might fall here somewhat into the middle, as they list as aging inducers via conferring DNA damage, for example, in skin and for mesenchymal stem cells (Andrade et al. 2012; Borodkina et al. 2014), while at the same time they might also play a more general role in signaling as well as for protein damage (Ray et al. 2012).
Genetically, data derived from studies ranging from yeast, C. elegans, D. melanogaster, and the mouse have shown that alterations in specific signaling pathways can limit stem cell function and induce aging. This in itself is a remarkable finding, as it implies that there are evolutionary shared mechanisms of stem cell aging, so that not each individual types of stem cell in a distinct organism ages at its own pace and mechanism. Specifically, alterations in developmental pathways – e. g., Wnt signaling (Brack et al. 2007; Liu et al. 2007; Takashima et al. 2008; Florian et al. 2013) or Notch signaling (Ohlstein and Spradling 2006; Biteau et al. 2008; Florian et al. 2013) – have been associated with decreasing stem cell function during aging. Furthermore, Wnt signaling has been linked to Klotho, a factor that is involved in the suppression of various aging phenotypes (Liu et al. 2007). Activation of Notch signaling initiates specific changes in the chromatin-modifying gene regulatory complexes, stem cell maintenance, and cell cycle progression (Campbell et al. 2008; Salat et al. 2008). In muscle precursor cells, activity of the Notch signaling pathway seems to be critical for regeneration (Conboy et al. 2003). In addition, a functional role of p21 has been established in activating a forward loop stabilizing senescence cell cycle arrest (Passos et al. 2010). An upregulation of the p16Ink4a cell cycle inhibitor has also been reported in aging organs, including human skin, and has been associated with reduced self-renewal of organ stem cells (Janzen et al. 2006; Ressler et al. 2006), while the NF-kB pathway has been shown to be closely related to inflammation and aging (Donato et al. 2008).
Another signaling cascade that has a major role in control of longevity is the insulin signaling pathway (Dillin et al. 2002; Bartke 2008), together with its sibling and hallmark of aging, metabolic regulation of (stem) cell function, as has been demonstrated, for example, in the D. melanogaster intestinal stem cell model system (Biteau et al. 2010; Guo et al. 2014). There is emerging evidence that these pathways are also affected by DNA damage (Niedernhofer et al. 2006; Mueller et al. 2014) and might be connected to the immune system (Guo et al. 2014) and that they control maintenance of pluripotency of stem cells (Bendall et al. 2007).
Changes in proteostasis (which refers to the control of the biogenesis, folding, trafficking, and degradation of proteins) and autophagy (elimination of proteins from a cell) have been also associated with aging, with the underlying paradigm that an accumulation of nonfunctional proteins over time in a cell will result in senescence or aging. Defects in proteostasis have been linked to diseases that are usually age associated like cardiovascular and neurodegenerative disorders (Warr et al. 2013; Brehme et al. 2014; Zaglia et al. 2014). Changes in proteostasis and autophagy with aging in stem cells though have been not investigated in great detail, with human ES cells, intestinal as well as hematopoietic stem cells being the current focus of investigation (Vilchez et al. 2014).
To further link all these cellular and genetic pathways to age-associated clinical phenotypes, it will be crucial to clearly assess elderly patients with up-to-date geriatric assessment technology to avoid mixing different variables, such as disability, frailty, and comorbidity (Fontana et al. 2014).
These data in aggregation indicate that in general cell extrinsic as well as stem cell-intrinsic mechanisms can contribute to age-related decline in stem cell function and that most listed hallmarks and their underlying molecular pathways might contribute in one or the other way to stem cell aging. The causes leading to molecular alterations in aging stem cells and in the stem cell environment are not completely understood. Yet the questions of which hallmarks of stem cell aging and of which cellular phenotypes are causally affected by which of these primarily genetically tested molecular pathways is still not know in detail, ultimately rendering the search for rational and targeted interventions to achieve attenuation of stem cell aging or even better rejuvenation very difficult. Again, as mentioned before, one central prerequisite to achieve stem cell rejuvenation is that the unwanted changes associated with aging of stem cells are actually reversible or not causally involved in maintaining the aged status.
16.2 Rejuvenation or Attenuation of Stem Cell Aging
The underlying causes leading to molecular alterations in aging stem cells and in the stem cell environment are not completely understood and most likely a combination of factors contribute to stem cell aging, as discussed so far in the different chapters of this book. Nevertheless, as already stated, the number of these distinct factors or pathways seems to be not open-ended but rather limited in number. Amelioration and rejuvenation of stem cells are usually addressed in mammalian systems, due to the translational aspects of this kind of question and the somewhat delayed attention that aging of somatic stem cells attracted in other model organisms. It has been reported that aged muscle stem (“satellite”) cells, in which the aged phenotype is a response to Wnt signaling, can be activated to differentiate and regenerate muscle in aged animals as efficiently as young muscle stem cells – either by forced activation of Notch or by factors in serum from young animals supplied by parabiosis (Conboy et al. 2003, 2005; Brack et al. 2007; Liu et al. 2007). In old mice, exposure to a young systemic environment increases activation of the Notch pathway improving satellite cell function and muscle regeneration (Conboy et al. 2003, 2005). Interestingly, parabiosis also increased the dendritic spine density of mature neurons and improved synaptic plasticity in the hippocampus of aged heterochronic parabionts. At the cognitive level, systemic administration of young blood plasma into aged mice improved age-related cognitive impairments in both contextual fear conditioning and spatial learning and memory (Villeda et al. 2014). Especially growth and differentiation factor 11 (GDF11), a member of the TGF-beta superfamily of cytokines, recently draw a lot of attention with respect to parabiosis as one of the factors found in the blood from young mice and reduced upon aging that when given to aged animals reverted both age-related cardiac hypertrophy as well as age-related dysfunction of mouse skeletal muscle. At least in the case of reverting aging-associated function of skeletal muscle, it was shown that GDF11 results, among others, in functional improvement of satellite cells and reduced markers associated with replication fork stalling (Flach et al. 2014; Sinha et al. 2014). In addition in cardiac and skeletal muscle, GDF-11 was also found to enhance vascular remodeling in the brain and by this means enhance neurogenesis in aged mice (Katsimpardi et al. 2014). Parabiosis and GDF-11 though seem to act to a great extent on more differentiated cells, especially in the case of the neuronal and cardiac system, as for these tissues very active contribution of stem cells to tissue homeostasis has not been reported, and as a consequence, rejuvenation should be driven by differentiated cells. The molecular mechanisms though of the action of GDF-11 are not known yet in detail.
It was further reported that deletion of components of the DNA damage checkpoint (Exo1, p21) attenuated loss of stem cell function, organ maintenance, and lifespan of telomere dysfunctional mice without increasing the cancer risk in vivo (Choudhury et al. 2007; Schaetzlein et al. 2007), but pharmacological targeting of these checkpoints is currently difficult and would need to be further developed.
Caloric restriction (CR) has been shown to extend lifespan under certain circumstances in most model organisms, although recently the effect of CR in nonhuman primates has been more critically discussed. Effects of CR can be seen in multiple, also stem cell-based tissues; thus, it most likely also affects aging of somatic stem cells, especially given that the molecular pathways that are linked to CR, like IGF-1, insulin, mTOR, and leptin signaling, have been all shown to affect stem cell behavior (Mazzoccoli et al. 2014). Surprisingly though, analyses on whether CR actually attenuates or rejuvenates stem cell aging are still rare, and only recently it was reported to have rejuvenating effects on murine hematopoietic stem cells (Cheng et al. 2014). In D. melanogaster, CR was found to attenuate the age-related decline in the function of the male germ line stem cell, which contributed to an extension of the reproductive period of flies when subjected to CR (Mair et al. 2010), while short-term CR enhanced skeletal muscle stem cell function in mice (Cerletti et al. 2012).
In this context rapamycin (which targets the mTOR pathway) has also been listed as a drug to halt or even revert stem cell aging. In the light of the finding that rapamycin has been one of the few drugs that resulted so far in lifespan extension when given to “normal” mice (Harrison et al. 2009; Neff et al. 2013) and which effects are also already measured in yeast (Powers et al. 2006), rapamycin has been implied in attenuation or rejuvenation of aging of in two distinct types of stem cells (Blagosklonny 2010), while the function of rapamycin on stem cell in vivo in longevity studies has not been performed yet. To note, it is likely that side effects associated with rapamycin treatment might preclude its translation into the clinic (Lamming et al. 2013).
In summary, these studies provide a “proof of principle” that reversion of molecular alterations in aging stem cells represents a promising therapeutic approach to improve organ maintenance and function during aging and that interventions targeting both the molecular and the cellular level might be successful and clinically relevant.
16.3 The Paradigm: Rejuvenation of Hematopoietic Stem Cells (HSCs)
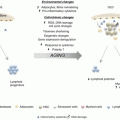
HSCs from young and aged mice differ in their function. Aging exerts a deleterious effect on HSCs self-renewal and differentiation ability, and HSC aging is driven by both intrinsic and extrinsic factors (Geiger and Van Zant 2002; Geiger et al. 2005; Rossi et al. 2005; Rando 2006; Ju et al. 2007; Mayack et al. 2010). Aged HSCs show reduced self-renewal activity determined in serial transplant assays (Janzen et al. 2006). When aged HSCs are transplanted together with young HSCs into lethally irradiated young recipients, aged HSCs are on average twofold less efficient in contributing to hematopoiesis compared to young HSCs (Morrison et al. 1996; Chen et al. 2000) and exhibit a twofold reduced ability to home to the bone marrow (BM) (Liang et al. 2005). Aging also affects the differentiation potential of HSCs. Aged HSCs are deficient in their ability to support erythropoiesis, and aged HSCs do not efficiently generate B-lymphoid progeny at the expense of increased myeloid cell lineage output. Aged HSCs exhibit distinct whole genome expression signatures (Rossi et al. 2005; Chambers et al. 2007) and increased double-strand breaks as detected by increased levels of gammaH2AX staining, a surrogate marker for DNA double-strand breaks (Rossi et al. 2007). Our data show that aged HSCs are less efficient in their ability to adhere to stroma cells and exhibit significantly elevated cell protrusion activity in vivo, reducing the time for effective interaction with the microenvironment (Xing et al. 2006; Geiger et al. 2007; Kohler et al. 2009). Thus, a defined canonical set of features phenotypically separates young from aged HSCs. It might be for this reason that research to improve aging of hematopoietic stem cells has gained momentum, as based on these phenotypes the level of attenuation of stem cell aging can be quantified in more detail and determined in more standard experimental settings compared to other stem cell systems.
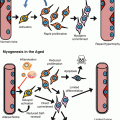
While multiple cell-intrinsic mechanisms for aging of HSCs have been discussed and summarized extensively elsewhere (refer to reviews by several authors (Geiger et al. 2013) (Snoeck 2005; Kamminga and de Haan 2006; Geiger et al. 2007; Rossi et al. 2008; Waterstrat et al. 2008; Beerman et al. 2010; Wang et al. 2011), the causative molecular mechanisms of HSC aging remain still largely unclear. We have recently identified a novel candidate mechanism of HSC aging the loss of stem cell polarity. This loss of polarity is induced by elevated Cdc42 activity driven by stem cell-intrinsic elevated expression of Wnt5a upon aging (Florian et al. 2012, 2013) and affects both proteins within the cytoplasm like Cdc42 itself and tubulin but also epigenetic markers in the nucleus, for example, the polar distribution of the acetylated form of histone 4 on lysine 16 (H4K14Ac). Cell polarity has been well characterized in epithelial cells (planar cell polarity) and neuronal stem cells, but not so far in HSCs. One intensely debated paradigm holds that asymmetric distribution of cellular components at HSC division and, subsequently, in the daughter cells determines their fate. Such an asymmetric distribution of cellular components has been shown by single-cell immunostaining (Beckmann et al. 2007; Rajendran et al. 2009) and in a fluorescent Notch-activity indicator system (Wu et al. 2007). It is not clear though whether polarity directly determines the mode of division and/or cell fate. Supporting a determining role for polarity in stem cell aging is a recent study showing that a loss of proper polarity in aged Drosophila germ line stem cells correlates with their reduced functions (Cheng et al. 2008). Given the established role of the small Rho GTPase Cdc42 in HSC polarity and differentiation, we postulate that Cdc42 may coordinate cell polarity and division symmetry in HSCs and that an altered mode of division contributes to stem cell aging. Most interestingly, pharmacological inhibition of the aging-associated increase in Cdc42 activity ex vivo resulted in rejuvenation of aged HSCs, as indicated by the reversal of almost all the phenotypic parameters associated with aged HSCs in transplantation experiments, including the level and position of H4K16 acetylation and cell polarity (Florian et al. 2012). The data thus support an important role for a correct cytosol and epigenetic polarity for stem cell function. Along the same line, genetic inhibition of the elevated levels of Wnt5a, a member of the non-canonical Wnt signaling network, in aged HSCs resulted, via reduced activity of Cdc42, in rejuvenation of aged HSCs and restored the youthful dependence on canonical Wnt signaling of HSCs (Florian et al. 2013).
Besides these approaches, attenuation of HSC aging has been achieved by lifelong caloric restriction in Balb/C inbred mice (Chen et al. 2003) or antioxidative therapy with NAC (Chen et al. 2009), which improved the self-renewal capacity of aged HSCs as tested in serial transplantation assays, thereby leading to partial or segmental HSC rejuvenation. Inhibition of mTOR activity, which has been reported to be elevated in HSCs from aged mice, both in vivo and ex vivo by rapamycin restored the self-renewal and hematopoiesis of HSCs, most likely via suppression of stem cell senescence, and enabled effective vaccination against a lethal challenge with influenza virus. These results imply that rapamycin, via inhibition of mTOR, has the ability to rejuvenate aged HSCs (Chen et al. 2009; Luo et al. 2014). In this context an interesting finding is also that iPS cells generated from aged HSCs, when reintroduced into blastocyst, give rise to young HSCs, establishing that aging of HSCs might be fully reversible (Wahlestedt et al. 2013). Therefore, aged HSCs can be fully rejuvenated, and the causal mechanisms of HSC aging do not irreversibly alter HSCs. Very recently also proteostasis was identified as a valid target to genetically rejuvenate aged HSCs. A regulatory branch of the mitochondrial unfolded protein response (UPR(mt)) is mediated by the interplay of SIRT7 and NRF1 and is coupled to cellular energy metabolism and proliferation. While SIRT7 expression was reduced in aged HSCs, SIRT7 upregulation improved the regenerative capacity of aged HSCs defining the deregulation of a UPR(mt)-mediated metabolic checkpoint as a reversible contributing factor for HSC aging (Mohrin et al. 2015). Whether these pharmacological and genetic pathways of rejuvenation are interdependent, as one would assume, has to be further investigated.
In aggregation, these observations support the notion that attenuation or even rejuvenation of aging of HSCs can be achieved by genetic as well as pharmacological targeting of cell-intrinsic mechanisms. Although such observations imply that reversal of the cell-intrinsic parameters of aging may be sufficient to achieve rejuvenation, HSC extrinsic factors, such as circulating cytokine levels and niche-specific factors, should perhaps also be targeted, especially in the context of rejuvenation in vivo. Mechanisms of HSC rejuvenation might also serve as a blueprint or template for testing similar rejuvenation interventions in other stem cell systems.
16.4 Rejuvenation of Stem Cells and Longevity
A key challenge in aging research is to confirm that molecular mechanisms of aging in model organisms are relevant for human aging. Another obvious question stemming from stem cell aging research is whether rejuvenation of stem cells will influence longevity. So far, there are no reports published that unequivocally determine that aging of a distinct type of stem cells limits lifespan, with the exception of some work on intestinal stem cells in D. melanogaster (Wang et al. 2014). Lifespan is most likely not limited by aging of just one single type of stem cell, and multiple parameters could be involved in determining longevity of the whole organism. On the other hand, drugs like rapamycin, which have been able to extent lifespan in mammals under certain circumstances, seem to affect multiple stem cell systems at once. Thus, targeting a critical and common pathway of stem cell aging, if that exists, might indeed serve as our fountain of youth.