Fig. 18.1
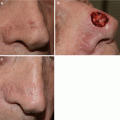
(a) Lentigo maligna on left cheek with brisk inflammatory response after a 12-week course of imiquimod. (b) Clinical clearance of lentigo maligna 4 months after completion of imiquimod course. (c) Durable clinical response 5 years after treatment
Case 2: Recurrent Lentigo Maligna After 12-Week Imiquimod Course
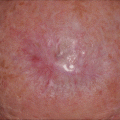
A 75 year-old Caucasian female presented with a pigmented lesion on the left nose and the initial biopsy showed a melanoma in situ. Woods light exam showed an asymmetric ill defined 2.6 cm pigmented patch in a background of extensive sun damage. After discussing the rationale, risks and benefits of staged excision versus nonsurgical options such as off-label use of topical imiquimod, the patient elected treatment with imiquimod. Patient began a 12-week course of topical imiquimod applied 5 times a week to the lesion plus 2 cm overlap area. She developed an exuberant inflammatory response to treatment (Fig. 18.2) with complete clinical resolution of the pigmented lesion. This clinical response was maintained until 1.5 years after treatment when she noticed repigmentation at the inferior aspect of the lesion. Two repeat biopsies both confirmed persistent melanoma in situ.
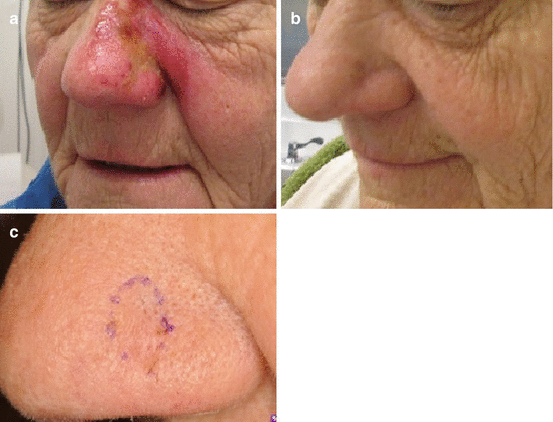

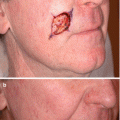
Fig. 18.2
(a) Lentigo maligna on left nose with an exuberant inflammatory response after a 12-week course of topical imiquimod. (b) 3 months post-treatment with clinical resolution of pigmented lesion. (c) Re-pigmentation at inferior aspect of lesion 1.5 years post-treatment that showed melanoma in situ on biopsy
Discussion
The objective of this case discussion is to highlight clinical pearls and practical considerations for selecting this treatment modality for select non-surgical cases.
These two cases illustrate the clinical application of topical imiquimod for off-label treatment of lentigo maligna and the variability of clinical response. In both cases, a 12 week treatment course (5 times weekly regimen) resulted in a brisk inflammatory response with subsequent clinical resolution of the pigmented lesion. However, similar initial clinical responses resulted in two different long-term outcomes. One patient had a favorable outcome with a durable response with greater than 5 years of follow-up while the other patient developed a recurrence of the lentigo maligna after 1.5 years.
This unpredictable variability in clinical response emphasizes the importance of close clinical follow up after topical imiquimod treatment. According to a review of 44 studies evaluating 327 tumors [1], lentigo maligna primarily treated with topical imiquimod has an average histologic clearance rate of 71.5 % (64.7–78.3 %) and average clinical clearance rate of 78.6 % (72.3–84.9 %) with a mean follow up of 34±11.8 months. The treatment regimens ranged from 3 to 5 days a week for 1–6 months. In general, the studies show a positive inflammatory response correlates with clinical and histologic clearance [1–5]. More rigorous treatment regimens (>5× a week or >60 applications) correlated with better outcomes as well [1].
The decision to start topical imiquimod as compared to surgery for facial lentigo maligna requires careful case-by-case evaluation of individual patient situations. Factors affecting treatment decision include age, co-morbidities, size and location of the LM, psychological and emotional state, and family and social support. Aside from the biological behavior of the lesion and host immune system, treatment success also depends on the patient’s willingness to complete the treatment course. Although the treatment duration varies from 6 to 12 weeks, longer treatment cycles may improve overall success.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree