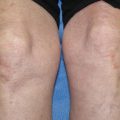
Fig. 4.1
Bullous pemphigoid. Typical clinical findings of tense bullae on an erythematous/urticarial base are seen on the upper back of this patient
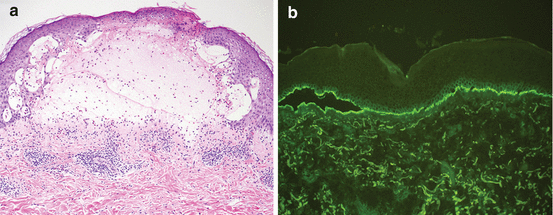
Fig. 4.2
Histology of bullous pemphigoid. (a). Hematoxylin and eosin staining showing a subepidermal blister with an inflammatory infiltrate demonstrating a predominance of eosinophils. (b). Direct immunofluorescence demonstrating C3 deposition along the dermal-epidermal junction (Images courtesy of Brian Swick, MD)
In the United States, the 1-year mortality rate has been estimated to be between 11 and 23 % [7, 9, 10]. A recent study showed the mortality of BP patients was no different to that of age-matched controls, suggesting the mortality of BP patients may be secondary to advanced age and comorbid medical conditions, rather than disease-specific issues [9]. However, these results conflict with other studies showing a higher mortality than expected with an age-matched population [7]. Overall, the major concern for patients is the significant morbidity secondary to pruritus and open wounds from blisters. Hence, the treatment is aimed at decreasing these symptoms along with facilitating healing to prevent secondary complications including infections.
There are currently no FDA-approved drugs for the treatment of bullous pemphigoid. The treatment of bullous pemphigoid is mainly based on case reports and physician experience. The most recent Cochrane Review, completed in 2010, identified ten randomized control trials with a total of 1049 patients [11]. All studies had different criteria for comparison with no placebo group. The evidence available in the literature regarding the effectiveness of steroid-sparing therapy will be examined below.
Topical Therapy
The evidence for high potency topical corticosteroids is favorable, although time intensive, and may be associated with poor patient compliance. The best evidence comes from a randomized, non-blinded multi-center trial of 341 patients with moderate to severe bullous pemphigoid that showed treatment with a potent topical corticosteroid (clobetasol propionate) was as effective and, in the “severe-disease” group, superior to treatment with oral prednisone (0.5 mg/kg/day for those with moderate disease and 1.0 mg/kg/day for those with severe disease) [12]. Among the patients with moderate disease, there were no significant differences between the overall survival, rate of control at 3 weeks, or incidence of severe complications between the two groups. However for those with severe disease, and treated with 1.0 mg/kg/day oral prednisone versus clobetasol propionate, the topical clobetasol was superior to oral prednisone with an overall increased survival and better disease control at 3 weeks. Severe complications were also higher in the group that received oral prednisone. It is important to keep in mind that this study was performed in an in-patient setting with nurses performing the application of topical steroids twice daily. These results may not be transferrable to those treated in an out-patient setting, and the time associated with the application of topical steroids to a large area would likely lead to poor patient compliance.
A follow up to this study, looking only at topical therapy regimens, was performed in 312 moderate to extensive BP patients. This study showed the standard regimen of 40 g daily of topical clobetasol tapered over 12 months was no better in controlling disease than the mild regimen of 10–30 g daily (depending on weight and disease extent) tapered over 4 months [13], suggesting that lower starting doses and faster tapers are appropriate.
A retrospective report of 96 patients, treated with clobetasol propionate found that 62 % were controlled with topical corticosteroids alone and only 25 % required adjunctive systemic treatment [14]. Other studies show rapid epithelization (4–17 days) in hospitalized patients with the use of high potency topical corticosteroids (clobetasol propionate) only, without any local or systemic side effects [15].
A single case series examined the use of topical tacrolimus for the treatment of BP and described two patients, on multiple oral medications, including oral prednisone. When topical tacrolimus was added, the oral prednisone was tapered, which was not possible before the addition of topical tacrolimus. However, the authors do note that topical tacrolimus is significantly more expensive than potent topical corticosteroids [16].
Systemic Corticosteroids
The evidence available for treatment with systemic corticosteroids suggests the type of steroid does not matter and a starting dose between 0.5 and 0.75 mg/kg of prednisone-equivalents is adequate to achieve control and remission. The randomized-controlled trial comparing topical and oral corticosteroids discussed above, showed prednisone dosed at 1.0 mg/kg/day did not have better efficacy and was associated with increased morbidity when compared with 0.5 mg/kg/day [12]. A randomized control trial looking at the initial starting dose of prednisolone 0.75 mg/kg/day vs 1.25 mg/kg/day did not show a statistically significantly difference in similar parameters between the two groups at 21 days. However, when a taper was initiated at half the initial starting dose, more patients in the high-dose group (55 % vs 33 %) were still free from all skin lesions [17]. A randomized control trial of 57 patients treated with methylprednisolone vs prednisolone at 1.0–1.5 mg/kg showed no difference in pruritus or number of bullae between the two systemic corticosteroids [18]. A retrospective review of patients treated with prednisone 1 mg/kg showed the time to suppression of blister formation was directly proportional to the number of blisters [19], suggesting that initial disease severity and the length of treatment with oral steroids, including the dosage taper schedule, may be more important than the starting dose.
Antibiotics & Niacinamide (Nicotinamide)
Looking at antibiotics alone, the evidence is limited. A retrospective review of 22 patients with BP treated with minocycline as adjuvant therapy showed a major response in six patients, a minor response in 11 and no response in five patients [20]. Thornfeldt and Menkes report two cases of men where their disease was resistant to potent topical steroids but cleared with tetracycline (250–1000 mg daily), one in conjunction with oral steroids. Remission was maintained on once daily oral tetracycline (250–500 mg) [21].
A single open-label study examined the use of oral erythromycin in fifteen patients with BP. All patients had contraindications to systemic corticosteroids or immunosuppressive therapy and were treated with 1 g erythromycin three times daily for 10–15 days [22]. Ten out of 15 patients responded to the erythromycin monotherapy in the initial phase of treatment and were continued on 1 g twice daily.
While there are no reports of niacinamide as monotherapy, there is evidence for niacinamide, used in conjunction with tetracycline antibiotics. There is one randomized, open-labeled trial comparing the combination of 500 mg of niacinamide three times daily, and 500 mg of tetracycline four times daily, versus prednisone therapy in 20 patients with bullous pemphigoid [23]. There were no statistically significant differences in response parameters between the two groups, although the numbers were quite small. The literature shows two additional case series and two case reports, encompassing a total of 13 patients successfully treated with tetracycline (500–2000 mg/day) in addition to niacinamide (1500–2500 mg/day) [24–27].
Dapsone
Venning et al. looked at 15 newly diagnosed BP patients treated with dapsone at a starting dose of 50 mg daily, increasing to 100 mg daily if no response was appreciated after 5–7 days. Five patients showed a complete response to treatment within 2 weeks, four showed no response and six were considered to be partial responders [28]. Bouscarat et al. retrospectively studied 36 BP patients treated with dapsone and 15 of those received dapsone as monotherapy (50–200 mg daily). Of those treated with dapsone only, 7 % of patients were considered complete responders and 20 % partial responders. Patients treated with dapsone in conjunction with topical or oral steroids showed a higher response rate [29]. As this study suggests, dapsone may have a role as adjuvant therapy in difficult to treat BP, but not as monotherapy. There is a single study of 13 patients with recalcitrant BP, requiring high doses of prednisone and azathioprine, treated with dapsone as adjuvant therapy (150–300 mg daily) with complete remission in 12 patients. Patients were more easily tapered off prednisone and maintained with statistically lower doses of prednisone, as compared to before dapsone was started [30]. These studies suggest that while dapsone could potentially be a non-immune suppressing alternative, it does not seem to be very effective, especially as monotherapy.
Azathioprine
Three randomized control trials, one cohort study and three cases series are available in the literature, examining the use of azathioprine to treat BP. Guillaume et al. took 100 patients with active BP and randomly assigned them to one of three groups: prednisolone alone (1 mg/kg/day), prednisolone + azathioprine (100–150 mg/day), or prednisolone + 4 large volume plasma exchanges. There was no difference in the number of patients in complete remission at 28 days or at 6 months and severe complications were highest in the group on azathioprine [31]. However, Burton et al. compared azathioprine plus prednisone with prednisone alone in 25 patients. This study concluded that azathioprine (2.5 mg/kg/day) reduced the maintenance dose of prednisone by 45 % without increased serious side effects or mortality [32]. Similarly, in a randomized control trial comparing oral methylprednisolone (0.5 mg/kg/d) plus azathioprine (2 mg/kg/day) with methylprednisolone plus mycophenolate mofetil (1 g BID), complete resolution and severe or life threatening events were observed to be similar in both groups. Time to resolution was quicker in the azathioprine group, although not statistically significant [33].
In smaller cohort studies and cases series, Ahmed et al. concluded combined therapy with azathioprine plus prednisone appears to be superior to prednisone alone in the treatment of BP in a series of 36 patients because the dose of prednisone was reduced by 50 % in people treated with azathioprine [34]. Greaves et al. used azathioprine in 11 patients on long-term prednisone and the prednisone was decreased or discontinued in all 11 patients; however he cautioned that corticosteroids should be used together with azathioprine during the acute stage since it has a slow onset of action [35]. A 4-year follow up of these patients showed 44 % remained in remission on azathioprine alone [36]. A small series (n = 5) of azathioprine as monotherapy showed a good response in four of five patients [37].
A systematic review published in 2011 reviewed the above seven published studies in which patients with bullous pemphigoid were treated with azathioprine and concluded that a level 2A recommendation was given to the use of azathioprine in combination with oral corticosteroids. No significant benefit of azathioprine with oral corticosteroids has been proven compared to monotherapy with oral corticosteroids, but combination therapy may be considered when the need exists for a corticosteroid-sparing effect [38].
Methotrexate
Most of the data available for methotrexate is as an adjuvant to either topical or oral corticosteroids. There is a single case report of a 90 year-old man successfully treated with low dose methotrexate as monotherapy that cleared his psoriasis and BP [39]. Further evidence from a single prospective study where 16 patients were treated with methotrexate as a first line therapy, showed 14/16 patients who did not discontinue methotrexate due to side effects achieved clinical remission by the end of the study period; in ten patients (62.5 %), topical clobetasol was added for severe pruritus [40]. The other evidence available is from case series. Dereure et al. studied 18 patients treated with high potency topical steroids for 2–3 weeks and an initial methotrexate dose of 7.5–10 mg/week with a maximum dose of 12.5 mg weekly. All patients showed a complete remission at 2 months, and all but one was maintained on methotrexate alone [41]. Heilborn reviewed 11 cases of patients over 70 years of age whose BP was not well controlled on potent topical steroids. Low dose methotrexate (5 mg/week) was initiated and increased to a maximum of 12.5 mg/week. All patients responded with decreased disease activity [42]. Bohm et al. reported on three successful cases using methotrexate as maintenance therapy after remission was achieved with topical or oral steroids [43]. A retrospective chart review found that five of eight elderly patients (average age 73.5 years) with treatment resistant BP, still active on oral prednisone, had clearance one month after adding low-dose methotrexate (5–10 mg/week), and all patients on methotrexate required lower doses of prednisone [44]. Another retrospective review reported 138 consecutive patients with a new diagnosis of BP. Ninety-eight patients were started on methotrexate 5 mg/week and 61 (62 %) were able to continue methotrexate monotherapy while 37 patients (38 %) benefited from the addition of prednisone. Only five of the patients treated with methotrexate had to discontinue the medication secondary to side effects, including gastrointestinal track irritation, anemia, increased liver enzymes and transient alveolitis [45].
Mycophenolate Mofetil
With the exception of the randomized control trial listed above comparing azathioprine and mycophenolate mofetil as steroid sparing agents, all other available data for mycophenolate mofetil in the treatment of BP is from case reports, including a total of five patients treated successfully with a dose of 2 g/day. In three of these patients, mycophenolate mofetil was used in conjunction with oral steroids as a steroid sparing agent. The systemic steroids were discontinued or tapered to physiological dosages over a period of 1–5 months without disease recurrence [46–48]. One report also described two patients treated successfully with mycophenolate mofetil as monotherapy without the usage of any systemic steroids [48]. In doses of 2 g/day, mycophenolate mofetil is generally well tolerated with gastrointestinal irritation being the most common side effect. A slightly increased risk of bacterial and viral infections and reversible myelosuppression have also been less commonly reported.
Cyclosporine
There are two published reports using cyclosporine for the treatment of bullous pemphigoid. Barthelme et al. retrospectively studied seven patients treated with cyclosporine dosed 6–8 mg/kg/day [49]. Four patients were treated with cyclosporine alone and two of these were considered treatment failures. Treatment was successful in all patients treated with corticosteroids in addition to cyclosporine during flares, but 2/3 patients relapsed after cyclosporine was discontinued. The main side effects were increased creatinine, which was reversible, and hypertension. Another case series reports successful treatment of two patients with cyclosporine (6 mg/kg/day) with a short clinical follow up. One was also on prednisone 20 mg daily which was tapered and discontinued 2 months after starting cyclosporine. The second patient was treated with 3 months of cyclosporine with disease remission for 2 months after discontinuation of the cyclosporine [50].
Plasmapheresis
Plasmapheresis has been a long-standing therapeutic option for the acute treatment of BP, usually in conjunction with systemic steroids with the first report of its success published in the French literature in 1979 [51]. This study described 12 patients treated with oral prednisolone (0.5 mg/kg) and four large volume (1.5 × theoretical plasma volumes) plasma exchanges over 2 weeks. Eight of twelve patients achieved disease control by 1 month. Five of these eight patients were able to successfully taper their steroids without a disease flare. Among the four patients who were not controlled on the initial treatment, all required an increase in prednisone up to 0.75 mg/kg (three patients) and up to 1 mg/kg/day with four additional plasma exchanges in a single patient [52]. After this initial study, a multi-center randomized trial was initiated. All patients received prednisolone (0.3 mg/kg), increased weekly if the disease remained active and 24/41 patients also received eight large-volume plasma exchanges over 4 weeks. The initial dose of prednisolone was effective in 13 of 22 patients receiving plasma exchange but in none of 15 patients receiving prednisolone only. Control of the disease was obtained with a mean daily prednisolone dose of 0.52 mg/kg in the plasma exchange group and 0.97 mg/kg in the other group [53]. As discussed previously, Guillaume et al. studied 100 patients with active BP who were randomly assigned to one of three groups: prednisolone alone (1 mg/kg/day), prednisone + azathioprine (100–150 mg/day), or prednisolone + 4 large volume plasma exchanges, and found no difference by adding plasma exchange to systemic steroids [31].
Despite these conflicting results, there are smaller case reports and case series that support the use of plasmapheresis to treat BP [54–61]. One study of 21 patients comparing steroids in conjunction with plasmapheresis versus steroids alone observed that adding plasma exchange had a lower rate of relapse at 6 months and required lower doses of steroids [61]. Egan et al. found plasmapheresis to be an effective steroid sparing therapy in their series of 10 patients. However, they did point out that due to its high cost and potential morbidity, plasmapheresis should not be recommended as first line therapy for bullous pemphigoid, and reserved for recalcitrant cases [55].
Immunoadsorption
Immunoadsorption (also termed immunoapheresis) is similar to plasmapheresis but specifically removes only immunoglobulin and immune complexes from patients’ circulation. While used in other parts of the world when an immediate decrease in circulating antibodies is desired, it is not currently approved for use in autoimmune blistering disease in the United States. Case reports show it can be used successfully in conjunction with oral steroids and other immunosuppressive medications with less treatment-limiting side effects than plasmapheresis [62].
Intravenous Immunoglobulin (IVIG)
Initial studies examining the use of IVIG for the treatment of BP showed unsuccessful results. Godard et al. reported a study of 11 patients with BP after previous treatment with oral prednisone who then received IVIG infusion [63]. Nine patients received 400 mg/kg/day × 5 days as monotherapy and two received 100 mg/kg/day and then 300 mg/kg/day for a total of 5 days. Two patients were also being treated with low dose prednisolone (0.2–1.0 mg/kg/day). One patient in the monotherapy group and both in the low dose IVIG group saw no improvement. The other eight patients saw rapid improvement in pruritus and reduction in development of new blisters.
Another case series of 15 patients with BP who experienced significant side effects on conventional therapy reported more successful results after treatment with IVIG [64]. All patients received clinical remission in 2–4 months of IVIG monotherapy, dosed at 2 g/kg given over 3 days every 4 weeks until no new lesions appeared. Then the interval between subsequent infusions was increased as tolerated. No serious side effects were reported. A case series looking at ELISA titers of ten patients treated with IVIG (2 g/kg given over 3 days every 4 weeks until healed) showed that treatment with IVIG caused gradual decline in BP180 and BP230 titers [65].
The rest of the available data comes from case reports totaling nine patients [66–71]. There is no uniform protocol or long term follow-up in these reports. However, three patients were considered treatment failures. The six patients who did respond to IVIg showed a steroid sparing effect in dosages ranging between 1 and 2 g/kg/cycle of IVIg administered every 2–4 weeks. Hence, it seems that IVIg can be an alternative treatment if oral conventional treatment is contraindicated or not tolerated, but needs to be administered every 4 weeks to maintain control of the bullous pemphigoid.
Rituximab
There are currently reports of 20 cases of BP treated with rituximab. Lourari et al. present five cases treated with rituximab 375 mg/m2 weekly × 4 weeks [66]. All five were being treated with topical steroids and two were also being treated with oral steroids (20 mg daily and 40 mg daily) and one with a second immunosuppressive agent (azathioprine). Three patients had complete remission of the BP. One had partial remission, and one patient with a history of ischemic cardiac disease died 10 days after the first infusion. One patient required a second course of rituximab 11 months later. Hall et al. treated seven patients with persistent disease activity on 17.5 mg or more daily prednisone, with rituximab dosed 1000 mg on days 0 and 14 [67]. All patients showed cessation of disease activity and tolerated the infusions without serious adverse effects. Six months after treatment, all patients were tapered to 25 % of their starting prednisone dose or 10 mg daily. Two patients did experience a flare of their disease at 7 and 11.5 months.
Of the other case reports, there were eight adults [68–73] treated with rituximab. Six were initially treated with oncology dosing of 375 mg/m2 weekly × 4 weeks, while two were treated with the rheumatology dosing of 1000 mg on days 0 and 14. Six of the eight patients were treated with concomitant systemic therapy. Four required a second cycle. One patient developed clostridium difficile enteropathy 4 weeks after the last rituximab infusion and subsequently died after developing hospital acquired pneumonia [73]. The other seven patients achieved complete remission with an average follow up time of 23 months.
Cyclophosphamide
There are concerns regarding treatment of bullous pemphigoid with cyclophosphamide, given the serious side effects of cyclophosphamide. In a retrospective study of ten patients treated with cyclophosphamide 100 mg/day, in addition to oral steroids, three patients died in the first 3 months attributable to side effects of the cyclophosphamide [74]. Four patients developed non-lethal bone marrow suppression and septicemia. However at the end of follow up, five patients did achieve clinical remission without additional treatment. Gaul et al. published a retrospective study of 20 patients with refractory BP treated with oral cyclophosphamide (50–100 mg/day) [75]. Eleven patients achieved complete clinical remission: eight patients on 50 mg/day and three on 100 mg/day. Four patients failed treatment and another patient self-discontinued treatment secondary to poor compliance. Twelve of 20 patients developed bone marrow suppression, but only three required discontinuation of therapy. One patient had intolerable gastrointestinal side effects and another died from heart failure that was not attributable to cyclophosphamide. A final case report of a woman with BP unresponsive to all other therapies reported success with pulsed IV dexamethasone (100 mg daily × 3 days) monthly and 50 mg daily of cyclophosphamide, without any treatment limiting side effects in 9 months of treatment [76].
Omalizumab
There is emerging evidence that specifically targeting IgE can provide symptomatic relief for patients with BP without the risk of side effects from broad immunosuppression. Omalizumab is a humanized monoclonal antibody which binds to IgE, preventing its binding to both the high and low affinity IgE receptors. It was originally FDA approved for moderate to severe asthma, but recently was approved in chronic urticaria, and studies suggest it may also be effective in the treatment of BP. Yu et al. published a case series of six patients with recalcitrant BP, unable to discontinue high-dose corticosteroids, treated with omalizumab. It was dosed using the asthma dosing nomogram, which is based on weight and serum IgE levels. Five of six patients responded to treatment with omalizumab without any serious adverse effects. Three used omalizumab as monotherapy and two others used it as a steroid-sparing agent in the induction and maintenance of remission [77]. Dufour and colleagues reported treatment of a 5 month old infant with severe, recalcitrant juvenile BP with omalizumab as monotherapy. New blister formation stopped after the initial injection and complete clinical clearing was achieved [78]. Another recently published case report of a 28 year-old male with BP who failed treatment with systemic steroids and daily cyclophosphamide, was significantly improved after two doses of omalizumab [79]. While initial reports are promising, further studies are needed with a larger cohort of BP patients to examine the long term response and compare omalizumab to other steroid sparing agents.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree