Fig. 6.1
Various clinical trial activities
You need to budget consistently. If your consent form states that a skin biopsy will be done, and there is no reimbursement for a skin biopsy in the budget, you will not be reimbursed. The informed consent should not state the subject’s health insurance or the subject will be responsible for the cost of the biopsy if it is study related.
You have to capture every possible expense related to a trial and account for it in the billing and budgeting process. If you don’t, it could hurt the financial viability of your investigative enterprise. Obvious expenses include all the procedures listed in the protocol flow chart. You should look at this carefully because there may be implied costs. For example, if the laboratory costs of a urinalysis and electrocardiogram are reimbursed, but the staff time for collection of the urinalysis or administration of the cardiogram are not reimbursed, the oversight needs to be addressed. You have to consider protocol-related items outside the study table. For example, if a specimen needs to be shipped, all shipping costs should be covered. If the shipping requires special containers or dry ice or overnight delivery, supplies and costs should be provided.
You have to think of your costs before you start the study. For example, you may need to budget your preparation for the study, your IRB fees, your setup costs for labs, radiology, and pharmacy. You can itemize these as start-up fees. You need to build in costs for the financial management of a trial, including your billing compliance costs (particularly if you use a clinical trial management system). You have to factor in end-of-study costs, such as storage of study documents and preparation for possible auditing.
It’s also important for you to determine how you will get paid. The timing and details can be a source of friction between investigative sites and sponsors (Fig. 6.2). There is often a long gap between committing your time and effort to being awarded a study to getting your first payment. Negotiating a first payment or an early payment (start-up payment) can help you with start-up costs so that you can support your staff in setting up the trial, completion of IRB submissions, reviewing your subject’s database and pre-screening patient’s medical records for eligibility.
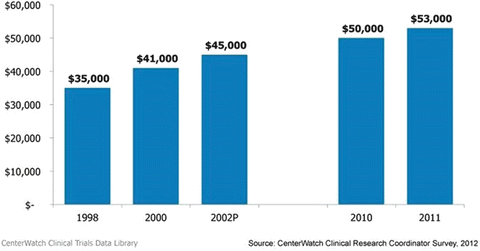

Fig. 6.2
Clinical research coordinator salaries (median annual salary, US$)
Be aware of the costs of data collection and management. Data may be collected on paper source documents and then submitted as paper or electronically via an EDC (electronic data capture) software system. While electronic data capture saves sponsors money, it takes extra study site time for data entry. There is no standardization. Your site will have to learn a different system for each study it participates in. The training time, and the time to enter data may cause your site to lose money simply on data collection and data entry. There is an effort to standardize EDC systems, for example, through the Clinical Data Interchange Standards Consortium, but until then, the panoply of software costs investigator sites staff time, money, and work.
Delays take time. Be sure to budget for them. One source of delays could be a use of “local” hospital or academic IRBs. Another is the budget and contract negotiation process, especially if it involves multiple parties, such as the investigator, CRO, billing department, IT, legal department, lab vendors, shipping vendors, and insurance providers (if applicable). This is especially true for large institutions. For example, at university sites, contract approval may require 20 steps and 13 decision points; and a community site may require much less time and have less steps. The fewer steps and fewer decision points, the faster contracting and budgeting can move forward. To start a cancer study at academic institution, the average start-up time is around 180 days, with about 100 days going toward negotiation. These times can be longer in Europe (200 days) and Asia (270 days). They are shorter in the USA for a central IRB (37 days) vs. a local IRB (107 days).
You need to know how many subjects are to be enrolled in the study. You have to take into account how many you will need to screen and how long it will take to achieve your goal. You have to see if the inclusion and exclusion criteria will alter your workload. You have to read to details of the study to see what is required of subjects. Is the study easy and straightforward, or is it laden with complex procedures and documentation at each visit requiring a great deal of your time and staff time? Are there many visits with impractical or inconvenient procedures and treatment regimens? Do the visits spill into extended hours such as nights, weekends, and holidays for which operating costs and staff costs are higher?
Laboratory costs to include in the budget are the tests and supplies and personnel time involved in obtaining specimens. You will need to include lab reagents, equipment maintenance costs, and a budget for packing and shipping specimens. If the shipping is international, you will need to budget for the extra costs and customs. If you have to store specimens, be sure to include the costs of refrigeration, alarm systems, and other back-ups.
You and your coordinator may need to travel to investigator meeting (90 % out-of-town) which could take a couple of days out of your schedule. Attendance at training sessions such as investigator meetings can be difficult. Many occur on weekends and take you away from family, but some occur during the week and may take you away from the clinic as well. Be sure to factor in the direct cost of travel and the indirect cost of lost revenue from your practice if training sessions or investigator meetings occur on one of your clinic days. Many sponsors will reimburse you for expenses of the meeting itself, but will not pay you for lost income. You should still include opportunity cost as a line item in your budget so that you can have a realistic assessment of the feasibility of conducting a trial. If your trial requires you to be away from your clinic for extended periods, and you lose a great deal of revenue in your absence, and it is not adequately reimbursed, you may have to reconsider doing the study.
Don’t forget to include study start-up costs. You need to add costs for additional storage space for study product or documents. Be sure to budget extra time spent on review of data, labs, assessment of subject progress, and adverse events. Adverse events in worldwide studies are more time consuming to review and address. If you have a number of sub-investigators you work with, be sure you include your time supervising them and communicating with them.
The recruitment phase costs 27 % of the budget, the most expensive and difficult part of drug development ($2 billion/year) (Fig. 6.3). Recruitment has become more difficult because the FDA has tightened criteria for efficacy, and because multiple companies are looking at the same pool of potential volunteer subjects. Also, sites may need to screen 10–20 subjects to identify one viable candidate, and sponsors don’t often compensate adequately for screening or screen failures.
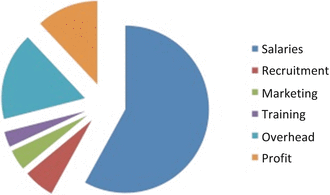

Fig. 6.3
Ask if the sponsor will help with subject recruitment. Some sponsors work with third party HIPAA compliant centralized study subject recruitment services. Ask if extended after-hours time will be required for your staff. You may also need to budget for this. If your sponsor requires special training (e.g., PASI training, electronic case report form training, special lesion counting training for the protocol), ask if this is reimbursed. Many studies also have source documents which are used to generate case report forms. Some sponsors provide them, some don’t. These can be a tremendous expense. If you are responsible for providing the source documents, be sure you budget for them. Your budget should include the actual cost of the documents and the time in preparing them, as each is study specific and study visit specific. If photography is required, find out if equipment and training are furnished.
While being a novice investigator, it is very important to conduct a successful study as well as maintaining the business side to make sure that you stay profitable. I suggest that when you are reviewing the protocol, you make a checklist to answer the following questions:
Are the number of subjects to be enrolled and timeline provided by the sponsor are realistic?
Would you have sufficient number of patients in your practice database to enroll in the study?
If you suspect that you do not have the sufficient number of patients in your own database; will you need to (a) advertise for patients; (b) do you need to collaborate with other practitioners in your area?
How difficult is protocol to implement? (I suggest carefully assessing labor intensity of the protocol and making sure sufficient well-trained staff is available)
Is proposed budget reasonable?
Most sponsors and CROs structure their budget on a fee for service basis; their proposed budget is per subject and prorated on a number of visits subject actually completes. Sponsors feel that they are contracting a service from investigator and do not expect to pay if services are not delivered. I would like to warn you about hidden cost that is usually associated with conducting a trial. For example, sponsor is proposing budget as mentioned above on per-subject basis; which does not include any study start-up activities including IRB submission; physician and coordinator time spent on initial protocol review and assessment.
The majority of the cost of a clinical research enterprise goes to staff salary.
My preferred way of coming up with a fair budget starts with careful review of the protocol required tasks and procedures including estimated time for investigators, coordinators, and sub-investigators. In addition, I take a careful look at the study required procedures: could they be all done at my clinic? Are there study procedures requiring radiology services? Please make sure to check with your local services on the cost of CT, or MRI required per protocol before signing off on the study budget. Remember to think about additional space to store the product or a need to have a pharmacist on the study. Give good consideration to extra time to be spent on daily/weekly review of each subject data, assessment of subject progress in the trial, or potential adverse events; and continuous communication with your study coordinator and sub-investigators.
Budget: Have you done a thorough budget with a cushion for unexpected problems or delays? You should negotiate a budget and the contract details before you make an IRB submission. Sponsors don’t like to discuss budgets until after you’ve already invested a great deal of your time. Some sponsors give you a nonnegotiable budget with little wiggle room. Sponsor budgets ignore many hidden costs of running a trial (Norm Goldfarb thinks up to 80 %). Most of the time is administrative (completing CRF, reading and processing correspondence, writing and sending correspondence, reviewing charts for potential subjects). Study visits account for only 20 % of the budgeted time. Employee benefits need to be included in the budget, which can add another 30 % to base pay.
Include all your known fixed costs.
Double your normal time for seeing patients, as study subjects and documentation of the study visit require more time.
Estimate the number of screen failures.
Estimate time for meeting with the IRB and other departments and for in-service training of PIs, SubIs, and staff.
Sponsors use PICAS (Pharmaceutical Information Cost Assessment Service) to generate budgets. The database includes procedure, institutional overhead, and personnel costs. The data are proprietary. Medidata charges are higher than PICAS. Kenneth Getz says that investigator compensation has declined 3 %/year, and work burden has increased 10.5 %/year.
Budget will vary depending on phase of the trials. Phases 1 and 2 studies are more complex than Phase 3. You need to account for this in your budgeting.
Negotiate in the higher range if you bring unique attributes to the study.
Consider nonmonetary factors: investigators may sacrifice 25 % of a grant to work with a novel or innovative compound, or may bend for a sponsor with an excellent reputation, or if a compound gives your patients a unique therapeutic opportunity.
Additional budget factors to consider: lab fees, shipping and handling fees, cost of dry ice, specimen collection fees, lab bookkeeping fees, pharmacy accountability fees, administrative time (contract negotiation 2 h, legal review 1 h, meeting with pharmacy, lab, nursing, IT 2 h, protocol revisions 1–5 h), IRB (sponsor usually pays for a central IRB), radiology studies, medical evaluations (estimate 1 h initial H&P and 1 h answering subject/family questions, consent, and study order; for study visits estimate 1 h coordinator activities and 1 h for data entry, and a half hour PI time). Add a half hour for each AE if mild, and 2–3 h for each SAE. For CRFs budget 8–10 h per patient, on average, double for a complex Phase 2 trial. Add several hours for query resolutions, and 1 h per patient for record archiving.
Don’t forget site sponsor meetings 1–2 h first meeting, investigator meeting 2–3 full days including travel, 1–2 h initiation meeting longer for coordinator, monitor visits 1 h PI 2–3 h coordinator, closeout visit 1 h PI 2–3 h coordinator, and audits half day for PI, 1–2 days for coordinator if in house, anybody’s guess if FDA.
Start-up fees: PI time for protocol feasibility and reality testing, IRB preparation time, recruitment plan development time, screen failures.
Miscellaneous fees: patient time and travel ($25–50 typical), study marketing fee (discussing the study with local practitioners, advertising, storage, overhead, unanticipated costs (protocol amendments, high screen failure rate, changes in charges from suppliers).
There are other techniques for budgeting. For example, you can budget by activity such as: medical evaluation; administrative and overhead costs; procedural costs; and one time fees. You can budget by evaluability. There can be a line item for each evaluable subject, or one who drops out because of an AE, which is especially important for Phase 2 trials. There can be a line item for each supportive patient. A supportive subject is one who terminated early, but may be able to provide safety data.
Equipment and storage. Be sure to budget for unique equipment. This can include a setup for standardized clinical photographs. Specialized equipment needs to be calibrated and maintained. These operating and maintenance costs should be prorated for the duration of the study and should not be overlooked. If your study requires skin biopsies, include the cost of materials such as local anesthetic, syringes, needles, specimen vials, suture, sterile surgical supplies and instruments, suture removal kits, bandages, and dressing in your budget. You may need to budget for handling, packaging, and shipping specimens. If shipments are to international locations or require specialty materials transfer agreements or IATA certified personnel, packaging, and permissions, be sure to budget for them. Have a defibrillator and medical emergency kit on site, and train your personnel in basic CPR and defibrillator use. You may need to store documents and study items in a special facility, for example, temperature controlled, having limited access, and secure. Storage costs can vary by study and can add up quickly.
6.3 Contract
Negotiating a clinical trial agreement involves multiple parties, including the sponsor, the research institute, and the principal investigator. Some of the key issues in negotiating agreements include confidentiality, intellectual property, publication opportunities, reimbursement, and liability. A successful negotiation is one in which all parties are satisfied with the outcome.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







