Fig. 6.1
Gross anatomy of the adipose organ of adult female 129Sv mice. The subcutaneous and visceral depots were dissected and positioned on a template of the mice to show their location in the organism. The mouse on the left was maintained under warm conditions (28 °C for 10 days) and the mouse on the right under cold conditions (6 °C for 10 days). Note the visually evident transformation of the color of the organ due to the increase of BAT and decrease of WAT contained in the organ. The organ is made up of two subcutaneous depots: A: anterior (deep cervical, superficial cervical, interscapular, subscapular, axillo-thoracic) and F: posterior (dorso-lumbar, inguinal, gluteal) and of several visceral depots: B: mediastinal, C: mesenteric, D: retroperitoneal, and E: abdomino-pelvic (perirenal, periovarian, parametrial, perivesical). Bar: 1 cm. Reproduced from Murano I, Zingaretti CM, Cinti S. The Adipose Organ of Sv129 mice contains a prevalence of brown adipocytes and shows plasticity after cold exposure. Adipocytes. 2005;1(2): 121-30 with permission
Subcutaneous Fat Depots
Two main subcutaneous depots are present in small mammals. The largest subcutaneous depot is localized in the anterior part of the body (anterior subcutaneous depot: ASC). In the posterior part of the body at the inguinal level is the simple, but large, posterior subcutaneous depot (PSC). In addition, minor subcutaneous depots are located in the limbs (Lfat).
ASC
Its shape and location is very complex: the more consistent and large part of it is localized in a deep fossa between the two scapulae (interscapular area). From this central core several elongated bilateral projections reach the neck and the upper limbs. Those projecting to the neck reach superficial and deep levels (cervical fat). The superficial parts can be subdivided into anterior and posterior. These last are located under nuchal muscles.
Two bilateral projections reach the upper limbs. Two are superficial and two are deeply located among the dorsal muscles. These last deep projections reach the axillae (axillary fat) and are continuous with thin and large dorso-lateral prolongments.
PSC
This depot can be considered as a fascia that wraps around the origin of posterior limbs. Thus this fascia starts at the dorso-lumbar area and runs at the inguinal level to end at contralateral dorso-lumbar area. While passing at the pubis level, a large projection reaches the gluteal area. This depot is easy to distinguish as three bilateral areas: dorso-lumbar, inguinal, and gluteal areas. A large lymph node is usually found in a point that can be considered as the boundary between the inguinal and the dorsal area. In some cases, a second bilateral lymph node can be found at the level of the dorso-lumbar area.
Lfat
The limb fat depots are always small. They are located under the fascia that surround the limb, thus even if they can be considered subcutaneous depots it must be outlined that they have a close connection with muscles. In this respect their localization can be considered quite similar to those deep parts of the anterior subcutaneous depot described above (see below for histology implications). Two small depots are located per each limb: at the level of shoulder and elbow in the anterior limbs and at the level of tight and popliteal fossa in the posterior limbs.
Truncal (Visceral) Fat Depots
In the trunk of female mice there are two main depots: one is located into the thorax cavity between the lungs and one is located in the abdominal cavity with extension into the pelvis [4]. The first depot is therefore located in the mediastinum and we refer to it as mediastinic depot. The second occupies several areas in connection with abdomino-pelvic viscera and therefore we refer to it as abdomino-pelvic depot. Minor depots are located in extra-serosal areas of the trunk such as between the thoracic wall and the thoracic parietal pleura and between the abdominal wall and the parietal peritoneum. Other minor, but very important from clinical perspectives, depots are located into peritoneal folds: omentum and mesenteric depots.
The fat depots in male mice differ in the trunk. This location contains a large depot, called epididymal fat, which is entirely surrounded by peritoneal sheets in tight connection with the testis.
Histophysiology
The vast majority of the above described depots show a mixed composition of adipocytes. Thus, white and brown adipocytes are the normal parenchymal cells of most of the depots of the adipose organ [5].
White adipocytes derive their name from the fact that they occupy the white parts of the adipose organ forming white adipose tissue (WAT) and contain lipids (adipocytes). They are large cells showing a spherical shape. Their size is variable from the largest epididymal or inguinal (70–80 μm in diameter, mice at 28 °C) to the smallest omental or mesenteric (40–50 μm, mice at 28 °C). About 90 % of their volume is occupied by a unique lipid vacuole (unilocular adipocytes) composed by triacylglycerides. Their nucleus has a crescent shape due to the need to adapt to the shape of the lipid droplet and cytoplasm is thin and poor of organelles (Fig. 6.2).
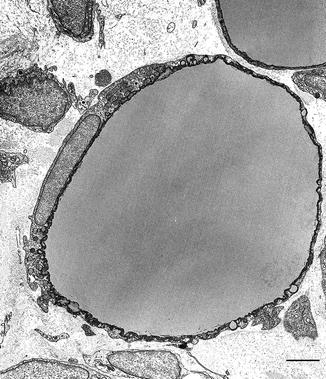

Fig. 6.2
Electron microscopy of unilocular white adipocyte from epididymal fat of three weeks old rat. Bar: 3 μm. Reproduced from Cinti S. The Adipose Organ. Milan: Kurtis; 1999 with permission from Editrice Kurtis
Brown adipocytes occupy the brown parts of the organ forming brown adipose tissue (BAT) and contain lipids (adipocytes). They are smaller cells (30–40 μm, mice at 28 °C) with polyhedral shape with roundish central nucleus and cytoplasm rich of numerous large characteristic mitochondria packed with cristae and several lipid droplets (multilocular adipocytes) composed by triacylglycerides (Fig. 6.3).
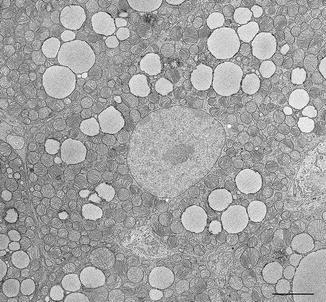

Fig. 6.3
Electron microscopy of multilocular brown adipocyte from interscapular fat of 10 days old rat. Note characteristic abundant mitochondria. Bar: 3 μm. Reproduced from Cinti S. The Adipose Organ. Milan: Kurtis; 1999 with permission from Editrice Kurtis
This different morphology accounts for their different function: white adipocytes store energy (triacylglycerides) in order to allow intervals between meals. Of note, sphere is the geometric shape allowing maximal volume in minimal space. Thus, most of the energetic needs of the organism during fasting are satisfied by fatty acids release of white adipocytes. White adipocytes are also endocrine cells because they release several hormones implicated in different metabolic pathways. The most important hormone released by white adipocytes is leptin [6]. Leptin signals the brain the amount of fat contained in the adipose organ. Low levels of this hormone induce hunger and behavioral changes to increase food intake.
Brown adipocytes burn energy (triacylglycerides) when animals are exposed to temperatures below thermoneutrality. The multilocularity favors a wide lipid-hyaloplasm interface allowing a massive disposal of fatty acids to be burned in the mitochondria [7]. Uncoupling protein 1 (UCP1) is uniquely expressed in the inner mitochondrial membrane of brown adipocytes and it is responsible for the main function of these cells [8]. In fact the enormous amount of fatty acids burned in the mitochondria is uncoupled from ATP synthesis and dissipated as heat. The process is tightly regulated by the nervous system through the sympathetic neurons that directly reach brown adipocytes with noradrenergic synapses en passant. Norepinephrine activates specific β3 adrenoceptors that are responsible for the activation of the thermogenetic program of these adipocytes [9, 10]. Recently, a hormone produced by brown adipocytes has been identified: betatrophin. This hormone is important for pancreatic beta cells proliferation implying a direct activity of brown adipocytes in glucose metabolism [11]. Fibroblast growth factor 21 is also produced by brown adipocytes with an important role on glucose metabolism [12, 13].
White adipose tissue differs from brown adipose tissue not only for their parenchymal cells (white and brown adipocytes), but also for vascular and nerve supply that is much more dense in the brown parts of the adipose organ [14].
As visible in Fig. 6.1, in animals kept at room temperature BAT is present mainly in ASC, in mediastinic and perirenal depots. Microscopic quantitative analyses of the whole adipose organ performed in different strains revealed that small amounts of brown adipocytes are also present in most of the white-appearing fat depots [5]. Thus, in normal conditions, all white fat depots contain brown adipocytes and would be better defined as predominantly white fat depots. Pure white depots are rare (epididymal). Mediastinic fat is the only depot we found composed exclusively by brown adipocytes in Sv129 mice kept at 28 °C.
Interscapular BAT is usually referred as “pure” brown fat. This is not correct from an anatomical point of view because it is only a part of ASC that clearly shows white and brown parts (Fig. 6.1). Together with the parenchymal adipocytes several other cytotypes are present in the adipose organ: blood vessels cells, nerve cells, fibroblasts, macrophages, mast cells, and adipose stem cells [1].
The adipose organ is considered one of the most important mesenchymal stem cell reservoirs of the whole organism. A simple method to isolate mature adipocytes from the rest of the tissue was developed about 30 years ago [15]. Collagenase digestion followed by centrifugation allows separation of mature adipocytes (floating at the end of centrifugation) from the rest of the tissue generally referred as stroma-vascular fraction (SVF). SVF contains all lipid-free cells including adipose stem cells. When seeded in primary culture with adipogenic medium, they develop into mature adipocytes [16]. These cells can also be forced to develop into muscle cells, condrocytes, and osteocytes under appropriate culture conditions [17]. The adipose stem cell origin is debated. For many years, cells related to the capillary wall of adipose tissues have been regarded as possible adipose stem cells [16, 18] and we recently pointed to endothelial cells as the source for the development of both white and brown adipocyte precursors [19, 20]. A recent paper reached different conclusions [21], thus the question remains unsettled.
Adipose Organ of Pregnant and Lactating Mice
During pregnancy and lactation the two subcutaneous depots (ASC and PSC) undergo a process of mammary gland development [1, 22]. In female mice at birth, the epithelial ducts end in three bilateral nipples located in the ASC and two bilateral nipples end in the PSC. During the first three postnatal weeks ducts develop infiltrating the whole subcutaneous fat depots. Thus, in female mice the ASC and PSC correspond to the structures that develop into mammary glands during pregnancy and lactation (Fig. 6.4). Under the hormonal stimulus of pregnancy and lactation (mainly: progesterone and prolactin respectively) new mammary gland components appear as lobulo-alveolar structures (or simply alveoli). These are the most important components of the mammary gland because they are the site for production and secretion of milk. They occupy all ASC and PSC volume among ducts. Lobulo-alveolar cells are characterized by large amounts of cytoplasmic lipids (Fig. 6.5). Thus they are parenchymal cells of the adipose organ in pregnancy and lactation. We identified the epithelial cells forming mammary alveoli as pink adipocytes. In fact the term adipocyte is used for cells that are characterized by abundance of cytoplasmic lipids (as in white and brown adipocytes). Considering then that white and brown describes the colors of the part of the organ containing white and brown adipocytes, pink is the color of ASC and PSC during pregnancy. Thus, even if alveolar cells form epithelial glandular structures their definition as pink adipocytes seems to be appropriate.

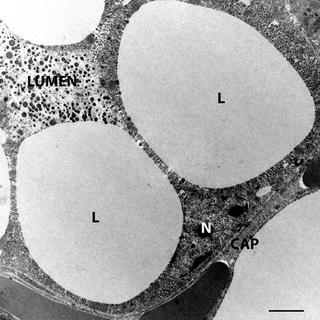

Fig. 6.4
Gross anatomy of the adipose organ of adult lactating female mouse. Note both subcutaneous depots are transformed into mammary glands. Bar: 1.5 cm. Reproduced from Cinti S. The Adipose Organ. Milan: Kurtis; 1999 with permission from Editrice Kurtis

Fig. 6.5
Electron microscopy of pink adipocyte. Note the abundant cytoplasmic lipid droplets (L). Several dense milk granules are visible in the lumen of the alveolus formed by pink adipocytes. N nucleus of pink adipocyte, CAP capillary. Bar: 2.5 μm
Plasticity of Adipose Organ
The presence of three different parenchymal cytotypes in the adipose organ raises the question: why are three different cells with specific well different functions contained in the same organ? With this question in mind we studied the adipose organ in different physiologic conditions in which one of the specific functions was enhanced.
Cold
Brown adipocytes’ main function is thermogenesis. Thus, cold exposure enhances the BAT activity. Looking at the gross anatomy of adipose organ of cold-exposed mice, we observed that most of the depots appearing as white in warm acclimated mice turned to a brownish color and most of brown areas became darker brown when animals were cold acclimated (Fig. 6.1).
Histology, electron microscopy, morphometry, and immunohistochemistry studies comparing the adipose organ of warm acclimated (28 °C per 10 days) with that of cold acclimated (6 °C per 10 days) mice showed that this browning of the adipose organ is mainly due to a direct conversion of mature white adipocytes into metabolic active thermogenetic brown adipocytes [23–25]. Of note, we also found intermediate forms of adipocytes never described before: paucilocular adipocytes. These adipocytes have a morphology similar to that of unilocular white adipocytes, but are smaller and show many small lipid vacuoles surrounding a predominant central lipid droplet. These cells contain numerous mitochondria with a morphology that covers a complete spectrum between classic “white” mitochondria and classic “brown” mitochondria. Furthermore, some of these adipocytes are immunoreactive for the protein marker of brown adipocytes: UCP1 (Fig. 6.6). These cells, that are also present in the adipose organ of warm acclimated mice, always occupy mainly the boundaries between white and brown areas in any fat depot. In summary, we think that under the noradrenergic stimulus due to cold exposure part of the WAT of the adipose organ converts into thermogenetic BAT by direct transition at cellular level from a specific phenotype into a different phenotype [26, 27].
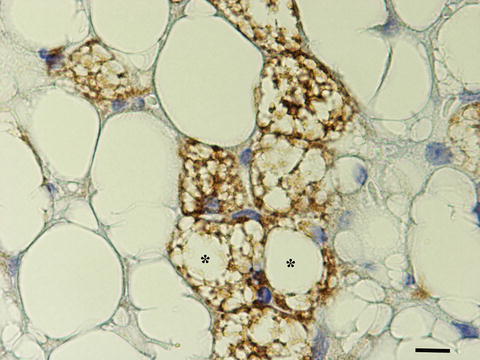

Fig. 6.6
Immunohistochemistry (UCP1) of subcutaneous fat of adult mouse exposed to cold (6 °C) for 5 days. UCP1 immunostained adipocytes (brown) are both multilocular and paucilocular (Asterisk). Bar: 18 μm. [4]
The key target for this phenomenon is the specific β3 adrenoceptor. Mice lacking this receptor have a very blunted phenomenon [28]. Sympathetic nervous system, activated by cold exposure, expands by branching its parenchymal fibers into the adipose organ and this is accompanied by white into brown conversion of adipocytes. In line with this observation, we found a positive correlation between parenchymal noradrenergic nerve fibers and the number of brown adipocytes in the adipose organ of two different strains of mice [3, 5, 29].
This white to brown plasticity of adipose organ is of paramount importance because mice lacking BAT or its function become massively obese in comparison to control mice with identical food intake and physical exercise [30, 31]. Furthermore, mice expressing UCP1 in white fat are obesity-resistant [32] and the obesity prone mice have reduced amount of BAT in comparison with obesity-resistant strains [5, 33].
BAT is also important to prevent diabetes and atherosclerosis. Removing the insulin receptor specifically in BAT induces hyperglycemia [34]. BAT explants regulates insulin sensitivity [35] and BAT activity controls triglycerides clearance [36, 37]. Thus, BAT can be considered as an important organ to fight the metabolic syndrome [37].
The recent re-discovery of BAT in the adipose organ of adult humans [38–42] renewed the attraction of scientists to this tissue in view of the human health care possibilities. We found that adult humans have BAT with most of the characteristics found in mice, including morphology, UCP1 expression, high density of parenchymal noradrenergic fibers, and perivascular adipocyte precursors. In our case series of human adults, as well as in other studies, age, body mass index and insulin resistance correlate inversely with presence of BAT in the classic anatomical site for humans: the supraclavicular area [42]. Age-related disappearance of human BAT seems to spare supraclavicular more than interscapular BAT [43], thus suggesting that supraclavicular BAT in humans correspond to interscapular BAT in mice even if detailed studies of supraclavicular BAT in mice are lacking. On the other hand, PET images showing metabolically active BAT in humans seem to suggest that this depot is tightly connected with subclavian vessels in line with the idea that BAT areas of the adipose organ are linked to heart, aorta and its main branches: carotid, subclavian, intercostals, and renal vessels. This is clearly visible in PET images of cold acclimated humans and corresponds to PET images of patients suffering from pheocromocytomas (benign tumors secreting high levels of epinephrine and norepinephrine) before surgery [44]. Thus, human adipose organ seems to share many characteristics of murine adipose organ and allows the hope we could modulate the browning of human adipose organ in order to pervert or curb the metabolic syndrome affecting a very large percentage of humans in western countries.
Of note, we recently showed that even human omental WAT, that seems to be a pure white fat, can be converted into BAT under the peculiar noradrenergic stimulus of pheocromocytomas. We found that in all 12 adult patients studied, omental WAT undergo a remodeling process: half of them had a significant reduction of the adipocytes’ size and half had a conversion of white adipocytes into UCP1 expressing brown adipocytes. Furthermore, this WAT to BAT transition was accompanied by a significant brown phenotype gene expression, and increased density of vessels and parenchymal noradrenergic fibers. Several UCP1 immunoreactive paucilocular adipocytes (we consider as morphologic-immunohistochemistry marker of direct WAT to BAT conversion, see above) were also found. Electron microscopy of paucilocular cells found in omentum of our case series of pheocromocytomas patients showed similar aspects of those found in fat of cold exposed mice: small lipid droplets at the periphery of a predominant central lipid droplet and numerous mitochondria with the white to brown morphology spectrum. We also found mitochondria with transitional characteristics: i.e. with white-like morphology at one extremity and brown-like morphology at the opposite extremity [45]. Similar mitochondria were described previously in adipocytes developed in vitro from the stroma-vascular fraction obtained from BAT, sampled from the axillary fat of a dead newborn [46]. Electron microscopy can distinguish perivascular adipocyte precursors [47, 48], thus it is possible to make quantitative analysis with this technique. Our quantitative electron microscopy showed that adipocyte precursors were not significantly increased in omentum of pheocromocytomas patients. In line with these results, the proliferative marker Ki67 was absent in nuclei of brown adipocytes confirming our previous murine data showing that noradrenergic stimuli do not induce proliferation of new adipocytes [45], consistent with results from other authors [49, 50]. All together, these data support the idea that brown adipocytes derive from a direct conversion of white adipocytes also in humans.
Beta adrenergic agonists are able to curb murine obesity [51–53] and drugs have been created in order to see if any therapeutic effect can be obtained to curb human obesity, however clinical trials were found to be unsuccessful [54, 55]. Nevertheless, several molecular mechanisms that overcome the beta adrenoceptors have been described recently (reviewed in [56]). Interestingly, physical activity seems to induce BAT activation and browning of WAT both in mice and humans [57–59].
Warmth and Obesity
When mice are exposed to warmth, a whitening effect is visible in the gross anatomy of the adipose organ [1]. Classic interscapular BAT changes the morphology of its brown adipocytes. They change gradually into white-like adipocytes. This morphologic transformation is accompanied by suppression of brown genes (such as UCP1) and activation of genes that are inactive in classic multilocular brown adipocytes (such as leptin and S-100b) [60, 61]. This transformation is also evident in beta-less mice (lacking all beta adrenoceptors). Beta-less mice are particularly prone to obesity and related disorders [31]. Genetically, obese mice have similar transformations of the adipose organ including brown to white transdifferentiation of interscapular BAT [62]. Thus, warm acclimation or chronic positivity of energy balance in absence of leptin or its receptor induces the opposite effect of cold exposure probably due to reduction of noradrenergic stimulus as suggested by knockout experiments described above. Chronic positive balance induces proliferation and development of new adipocytes and the adipose organ of obese humans can reach 60–70 % of the body weight (normal 15–20 %) [63, 64]. Thus the obese adipose organ is characterized by hypertrophy and hyperplasia of adipocytes.
Macrophage Infiltration
Two independent groups in the United States showed that the adipose organ of obese animals and humans is infiltrated by macrophages. This infiltration is correlated with the size of adipocytes and is strictly coincident with the appearance of insulin resistance. Cytokines, playing key roles in inducing insulin resistance, are expressed more by the stroma-vascular fraction of fat (including macrophages) than the floating fraction formed by mature adipocytes, thus implying the importance of macrophages infiltration in determining insulin resistance and subsequent type 2 diabetes [65, 66




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






