Fig. 14.1
Paget disease: in its early stage, PD appears as an erythematous, scaly, crusted lesion of the nipple
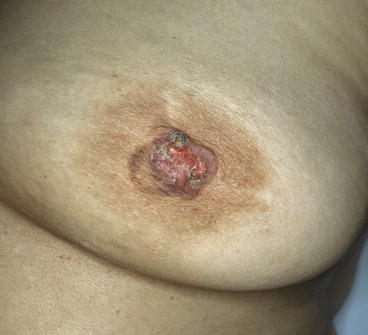
Fig. 14.2
Paget disease: in more advanced PD, the lesion has spread to the areola, and the nipple appears flattened
The eczematous status can temporarily be resolved with corticosteroid treatment. In addition, tiny vesicles can also appear and disappear on the nipple area. This adds to a delay in diagnosis. Typically, 10–12 months can pass before PD is correctly diagnosed. In the latter stages, PD can ulcerate and destroy the nipple or areolar complex. Bloody discharge, yellowish or bloody, is more common but only at this stage.
Assessment. Physical exam, mammographic X-ray and breast ultrasound are the first-line diagnostic steps in order to find any possible breast lesion or mass, which together with the clinical suspicion of PD can corroborate such a diagnosis. Physical examination can reveal a mass, which might then be seen as a nodule by mammogram or ultrasound. Even if neither masses nor nodules are palpable or visible, microcalcifications could be a possible finding seen at mammogram. It is important to consider that a concomitant tumour might be located several centimetres away from the nipple and areola complex. About 50–70 % of patients with biopsy-proven mammary PD show positive findings on mammography [1].
Ultrasound has no characteristic findings in PD, yet findings may include dilated irregular subareolar ducts, calcifications and a subareolar mass or flattening and/or thickening of the nipple.
MRI could be indicated in a dense breast or when an underlying DCIS or invasive cancer has not been identified by first-line investigations. MRI can also help in detecting multifocal/multicentric lesions and in better defining lesion topography with respect to the nipple-areola complex [1]. Any dubious breast lesion will be biopsied independently of PD suspicion.
Simultaneously, diagnosis of PD is established by cytology or wedge biopsy of nipple. For nipple sampling, there are several procedures including:
Surface cytology: a glass slide is leaned against the surface of the nipple many times.
Scraping cytology: the cutting edge of the glass slide is used to remove the top layer of skin.
Punch biopsy: a circular cutting tool (punch) is used to remove a disc-shaped piece of tissue (see Sect. 6.3.3).
Wedge biopsy: a lancet is used to remove a small wedge of tissue.
A mammogram should always be performed. However, it is usually negative in the case of a preinvasive cancer. It is important to consider that, in case tumour was present, that tumour could be located several centimetres away from the nipple and areola. In dense breasts, MRI could be indicated.
In the case of a negative cytology test for the nipple-areola eczematous lesion, if the PD suspicion remains high, a punch or wedge biopsy must be performed.
Typically, there are four possible clinical patterns of the disease:
Changes in the nipple or areolar complex only (true PD)
Changes in the nipple or areolar complex with an underlying breast mass
Clinical presentation of a breast mass with histologic confirmation of PD
Coexistence of typical PD with detached distant tumour (rare)
True PD is a variety of ductal carcinoma in situ (DCIS), associated to clinical eczema of the nipple and characterized by large, round “Paget cells” found in the epidermis of the nipple and the areola. The pathogenesis of PD still remains debatable: there are two theories about the origin of the Paget cells. In the first, it is thought that the cancer cells start growing inside the ducts within the breast and then make their way out to the nipple surface (epidermotropic ductal theory). This would appear to explain why so many people with the Paget disease of the nipple have an associated underlying breast carcinoma.
Another theory suggests that PD originates from the epidermis cells of the nipple itself (in situ malignant transformation theory). This theory would explain the small number of people who only have the Paget disease in the nipple or have a second that appears to be completely separate from the Paget disease.
In literature, the rate of associated underlying carcinoma of the breast ranges from 82 to 100 %, and in nearly 60 %, the carcinoma is a unifocal disease [1, 2].
A schematic diagram of clinical patterns of mammary Paget disease based on accepted concepts of pathogenesis is showed in Fig. 14.3.
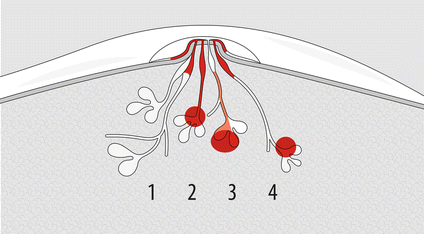

Fig. 14.3
Clinical patterns of mammary Paget disease based on accepted concepts of pathogenesis. 1 Paget cells, derived from epithelium of the main ducts, have an extension into epidermis of overlying nipple; no mass is found in about half of cases. 2 Epidermotropic lesion with detectable mass and spread of the tumour toward the surface and the depth (about one-third of cases). 3 BC with occasional histological finding of Pagetoid cells with minimal epidermotropic diffusion and usually without skin involvement (few cases). 4 Coexistence of typical PD with detached distant tumour (rare)
Primary treatment of PD is still surgical. However, due to conflicting theories of how PD develops, there is much controversy with regard to radical versus conservative surgical treatment. If the epidermotropic ductal theory is correct, lumpectomy or mastectomy are the treatments of choice. If the in situ theory is correct, a wide skin excision alone would be the most appropriate therapy in case of a disease involving a limited area.
Mastectomy has been considered as the standard treatment procedure for these patients for many years (Ashikari R) (Chaudary MA) (Yim JH). However, it is reasonable to consider breast-conserving surgery since similar long-term survival rates to mastectomy have been reported [3]. Several studies have already shown that breast conservation with radiation therapy is an oncologically safe option [4].
In patients with only nipple or areolar complex involvement, some experts recommend skin excision with consideration of adjunct radiation therapy. There have been some reports of using radiation therapy alone, with varying results. At present, radiation therapy alone is not considered a standard method of treatment.
Breast-conserving surgery that includes removal of the nipple and areola followed by whole-breast radiation therapy is a safe option for people with PD assessed as localized with some radiological changes in the retroareolar area (microcalcifications or suspicious opacities), without an underlying distant invasive cancer.
Mastectomy, associated with oncoplastic repair techniques to maximize cosmesis, is the treatment of choice in patients with distant or multicentric masses or with an extensive intraductal component or in those with relapses after conservative treatment.
Sentinel lymph node biopsy should be performed to evaluate the axilla when invasive BC is present [2, 5] and in any case of mastectomy given the possibility of an unrecognized invasive component. In a study, the frequency of positive sentinel lymph node biopsy was reported to be 11 % patients [6].
Adjuvant therapy and prognosis depend on a variety of factors, including the presence or absence of invasive cancer and, if an invasive component is present, on its stage. For patients with PD alone, as for DCIS, preventive treatment with tamoxifen could be discussed with the patient.
Prognosis. As treatment, prognosis depends on the presence or absence of palpable masses or axillary nodes and underlying invasive carcinoma. One half (50 %) of patients with PD presenting with a palpable breast mass have associated axillary lymph node metastasis. Two-thirds of patients with axillary node metastasis were reported to have a palpable breast mass.
Even in patients with mammary PD and no underlying tumour, 30 % may develop an invasive carcinoma at a later date, and 20 % of patients already have an associated in situ carcinoma of the breast. However, other reports indicate that no axillary metastases were detected in patients without a palpable breast mass.
Patients with an identifiable associated underlying breast tumour have a survival rate of 38–40 % at 5 years and a survival rate of 22–33 % at 10 years. Mortality for metastatic breast carcinoma in patients with mammary PD and underlying cancer is 61.3 %, with a 10-year cumulative survival rate of 33 %.
The reported survival rate of patients with PD without a palpable breast tumour (prior to surgery) ranges from 92 to 94 % at 5 years and from 82 to 91 % at 10 years [7].
14.2 Inflammatory Breast Cancer
Clinical Practice Points
Inflammatory breast cancer (IBC) is a rare disease that typically presents with a rapidly enlarging erythematous breast, a peau d’orange appearance and/or abnormal breast warmth, often with no detectable breast mass.
In the presence of IBC, defining the presence or absence of ipsilateral and contralateral disease in the nodal region, and defining the presence or absence of distant metastatic disease, is mandatory.
Treatment for IBC is usually more aggressive than treatment for most other BC. Preoperative systemic chemotherapy is the standard of care. The aggressive biology of IBC should encourage enrolling patients in clinical trials that are testing new treatments.
On note, a dimpled appearance of the skin, but without extensive redness, may be due to massive axillary node involvement in some advanced noninflammatory BC.
Incidence. This uncommon type of invasive BC accounts for about 1–3 % of all BCs. IBC has rapid and deceitful onset, often in a matter of weeks but also few months, and often lymph nodes are involved, as well as one or more distant sites.
Compared with noninflammatory types of BC, IBC tends to be diagnosed at younger ages with a mean age of 50–58 years. However, there are no validated risk factors that might aid in raising suspicion for IBC. Like other types of BC, inflammatory can occur in men, but usually at an older age than in women, with a median age at diagnosis of 66.5 years. IBC is also more common in obese women than in women of normal weight and in black than in white women.
The aggressive course of IBC, together with accumulating data on its unique molecular and epidemiological characteristics, lends support to the hypothesis that IBC may in fact be a distinct biological entity rather than a subtype on the spectrum of locally advanced breast cancers (LABC). To this end, it is important to distinguish two distinct clinical varieties of IBC that are commonly cited in the literature. The term primary IBC is used to describe the de novo development of IBC in a previously normal breast. In contrast, secondary IBC describes the development of inflammatory skin changes that mimic primary IBC either in a breast that already had cancer or on the chest wall after a mastectomy for non-IBC [8].
Clinical presentation. Diagnosis of IBC is mainly clinical. Patients with IBC usually present with a complaint of colour change in one breast, usually pink (Fig. 14.4) that evolves into a darker red (purple) and spreads over the entire breast. The patient might describe a sensation of heat in the breast, and the breast itself enlarges rapidly over a period of only a few weeks. Patients usually do not experience fever neither leucocytosis. The typical peau d’orange appearance of IBC stems from lymphatic oedema around deepened hair follicles.
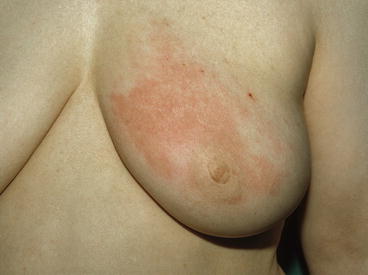

Fig. 14.4
In IBC, redness involves more than one-third of the breast, and the skin is usually thickened
Of note, a pitted appearance of the skin with peau d’orange, but without extensive redness, may be due to massive axillary node involvement in some advanced noninflammatory BC (Fig. 14.5). Some cases of moderate severity involving only a part of the breast are rather pseudo IBC. Peritumoral and cutaneous oedema is more often to be found beyond the limits of the tumour, even though at times, it is difficult to precise its limits. The enlarged lymph nodes are sometimes matted together and attached to the tumour by an indurated cord of neoplastic lymphangitis.
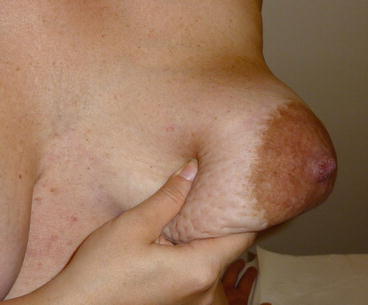

Fig. 14.5
A pitted appearance of the skin, with peau d’orange but without extensive redness, may be due to massive axillary node involvement, as in this 58-year-old woman. This case should not be considered as IBC
American Joint Committee on Cancer (AJCC) provides the current gold standard definition for this form of BC, describing it as a clinic-pathologic entity that is characterized by diffuse erythema and oedema (peau d’orange), often without an underlying palpable mass. The clinical presentation of IBC is due to tumour emboli within dermal lymphatic vessels, which may or may not be present on skin biopsy. Therefore, the AJCC relies on the clinical features of IBC and considers the pathologic features to be supportive of, but not necessary for, diagnosis.
Recently, minimum diagnostic criteria have been set forth by an international expert panel [9], which include:
History of rapid onset of breast erythema, oedema and/or peau d’orange and/or warm breast, with or without an underlying palpable mass.
History of flattening, crusting or retraction of the nipple may be present.
Patients may have a history of being diagnosed with mastitis not responding to at least 1 week of antibiotics.
Duration of history of no more than 6 months.
Clinical examination revealing erythema occupying at least one-third of the breast.
Clinical examination may reveal underlying palpable mass with or without palpable locoregional lymph nodes with or without nipple abnormalities.
Assessment. The key roles of imaging in IBC are to identify a primary breast tumour and facilitate image-guided diagnostic biopsy, stage locoregional disease, diagnose distant metastases and evaluate tumour response to neoadjuvant therapy [8]. So all women with a suspected IBC should undergo a mammography and an ultrasound of the breast and regional lymph nodes. Probability of axillary nodal involvement is 90 %.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








