, Paul N. Chugay1 and Melvin A. Shiffman2
(1)
Long Beach, CA, USA
(2)
Tustin, CA, USA
Abstract
Silicone is a generic, extremely broad term for materials that contain a backbone consisting of repeating silicon-oxygen atoms where each silicon atom also has two organic groups. Most silicone polymers can be placed into three general categories based on their methodology of curing or polymerization: addition cure (most common), condensation cure, and peroxide cure. The author discusses the various aspects of polymerization and design and manufacture of silicone implants. Also shown are the varieties of silicone implants used by Dr. Chugay.
Introduction
Biomaterials, as a category, have exploded over the past decade with new materials being developed and qualified for use in medical applications. Along with all of the new materials, silicone has maintained its position as arguably the best known and positioned as the gold standard. Silicone is a generic, extremely broad term for materials that contain a backbone consisting of repeating silicon-oxygen atoms where each silicon atom also has two organic groups (Fig. 1.1). These organic groups (-R) are most commonly methyl but may also be vinyl, trifluoropropyl, phenyl, or a myriad of other organic groups. By varying these groups, different physical and chemical properties may be conferred on the resultant polymer. Most silicone polymers can be placed into three general categories based on their methodology of curing or polymerization: addition cure (most common), condensation cure, and peroxide cure.
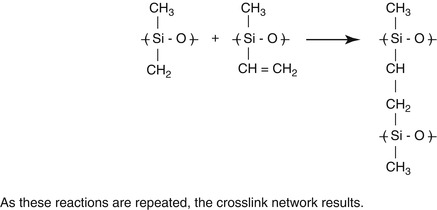

Fig. 1.1
Molecular formula for silicone
Curing or Polymerization
Addition cure utilizes the addition of a silylhydride to a site of unsaturation, normally a vinyl group. This reaction is catalyzed by a metallic compound, usually platinum. The catalyst may be present in a concentration range of 5–20 ppm, but most commonly is present at the lower end such as 7–8 ppm. Should multiple silylhydrides be preset in the same molecule, they will react with vinyl groups in the prepolymer creating a crosslinked network. Most silicone polymers used in medical devices are manufactured using the addition cure system. This system is routinely a two-part system where one part contains the platinum catalyst, vinyl functionality, and sometimes an inhibitor. The other part contains the silylhydride crosslinker as well as the presence of vinyl functionality on the silicone backbone. These two parts when mixed thoroughly can be pumped, poured, or injected into containers (molds) which are configured in the shape desired. There is a finite time during which this can occur. This is called the working time. Beyond this time, the mixture becomes too thick to work with and is unusable. Once in the container (mold), heat is then used to activate the mixture initiating the cure or vulcanization process resulting in an elastomer. These same basic sequences are used for easily flowable materials such as liquid silicone rubber (LSR) or thick, non-pumpable polymers such as high consistency rubber (HCR).
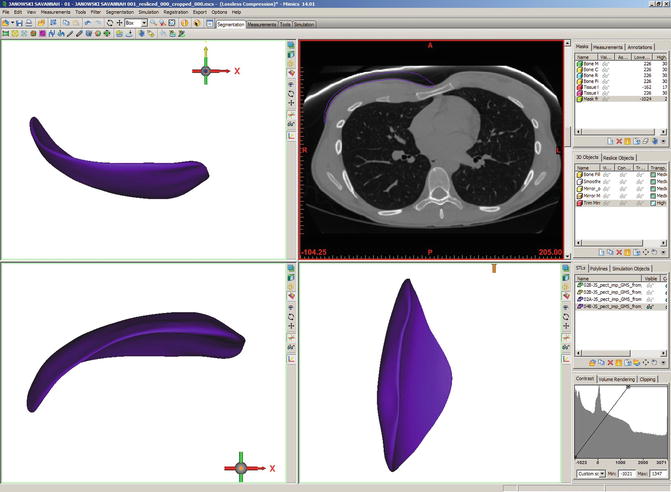

Fig. 1.2
Rendering of implant based on CT of patient presenting for pectoral augmentation
Condensation cure may be one- or two-part systems and utilizes hydrolyzable groups on both the crosslinker and polymer components. The one-part system is more common. When removed from their storage containers, they react with moisture from the atmosphere which diffuses through the polymer. This results in the hydrolysis of the reactive group on the crosslinker molecule. The silanol species formed can then react with a hydrolyzable group attached to the polymer end. When repeated on the same crosslinker molecule, the crosslink network results. These one-part systems are referred to as room temperature vulcanization (RTV). Though not used routinely in the primary fabrication of medical components, RTVs are commonly used as an adhesive to bond other silicone materials together. Two-part RTVs are occasionally used in the fabrication of medical parts where part thickness exceeds 0.2 in. (5 mm).
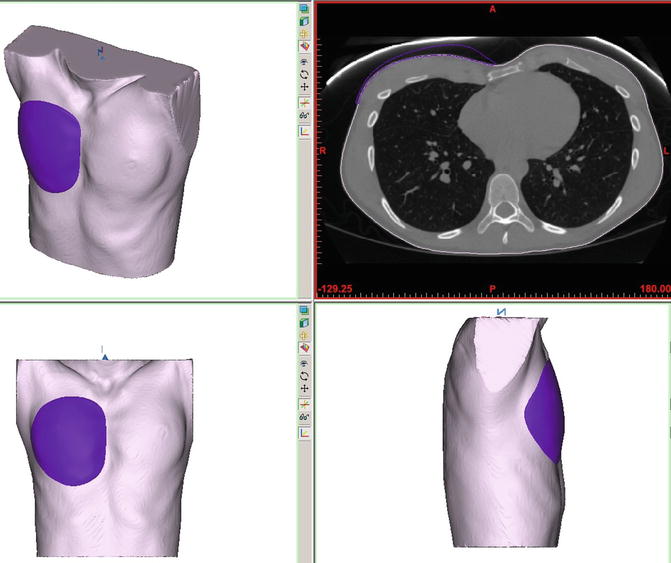


Fig. 1.3
Another view of rendering of implant based on CT of patient presenting for pectoral augmentation

Fig. 1.4
Clean room assembly
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








