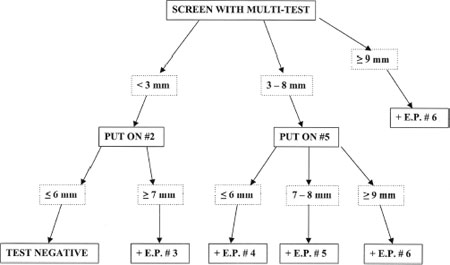
7 As previously discussed, intradermal dilutional testing (IDT) can be an effective and sensitive method for the diagnosis of inhalant allergy. It is a safe technique, in that it systematically assesses allergic sensitivity beginning with very dilute levels of antigen, and it sequentially progresses to stronger concentrations to quantify the degree of sensitivity. It has been employed successfully for the diagnosis and treatment of inhalant allergy for over a half century. Although IDT demonstrates good clinical efficacy, there are circumstances in which the use of complete dilutional testing might not be indicated. Time constraints, personnel availability, and economic factors often require compromises in testing. Because quantitation in testing provides advantages in safety and in accurately determining starting doses for immunotherapy, it is useful for any testing technique to provide results indicating the degree of antigen sensitivity, within the constraints of practical issues in staffing, time, and cost. One method that has been designed to provide this effective compromise is modified quantitative testing (MQT). The MQT method was first described in 2003, where it was presented as a blended method of prick and intradermal techniques that was developed to yield “an estimate of the strength of the allergic response that can be interpreted using standard [IDT] nomenclature and used in vial preparation.”1 MQT was described in greater detail in a monograph published later that year.2 Since being introduced in the early 2000s, MQT has become increasingly used as a method for testing for inhalant allergy, and it has proven to be both sensitive and specific in clinical practice. MQT is a blended method for the assessment of inhalant allergy. It initially uses prick testing to estimate an approximate range of skin reactivity to individual antigens, and then it employs one specific intradermal test for each antigen at a predetermined dilution to further refine and quantify the degree of allergic sensitivity. Prick testing devices and methods vary in their clinical measurement properties. MQT generally employs more current, multipronged testing devices such as the Multi-Test II device (Lincoln Diagnostics, Inc., Decatur, Illinois) or the Quintest device (Hollister-Stier Laboratories, LLC, Spokane, Washington) because they produce testing results that are more accurate and reproducible than the single-stick devices.3,4 As with all forms of skin testing, safety is essential. The patient is questioned about his or her medical history, including prior testing, asthma, and history of anaphylaxis. In addition, the patient is asked about medications that may interfere with skin testing (e.g., antihistamines, tricyclic antidepressants) and medications that may increase the risk of testing (e.g., beta-blockers). Once the history is complete, the testing is described to the patient and his or her agreement to proceed is obtained. At this point the upper and lower arms are exposed and are cleansed with alcohol or another acceptable agent for sterilization. In performing an MQT battery, it is important first to ensure that the skin is able to respond to antigen placement, as with all forms of skin testing. A histamine control is used to check responsiveness, and it can be placed in one testing well of a multipronged tray. An alternate approach that can be used to assess skin responsiveness is the placement of a No. 3 dilution histamine intradermal wheal, as would be used for IDT. Because a No. 2 glycerin wheal must also be placed to allow interpretation of the intradermal tests, both intradermal wheals can be raised at the same time prior to prick testing. It is also important to include a saline control in one well of the prick-testing tray, as a small number of patients have a dermatographic response to the prick test and demonstrate whealing as a result of the mild trauma of prick test placement. If skin responsiveness has been demonstrated through a positive histamine control, prick tests are placed on the volar forearms using the multipronged testing devices as described in the preceding chapter. After placement, 20 minutes are allowed to elapse for development of positive whealing. The placement of a subsequent intradermal test is based on the size of the wheal produced by prick testing. The degree of sensitivity is estimated from the blended responses of both the prick and intradermal test results, and a quantification of this sensitivity is made using the same endpoint (EP) system defined in IDT. In MQT testing, a wheal size of 3 mmin diameter defines a positive skin response. The patient is assumed to be sensitive to that antigen, and further testing will be necessary to refine the estimate of the degree of sensitivity. If the patient has a very vigorous response to prick testing, which is defined as a wheal of 9 mmor greater in diameter, the patient is considered to have a maximal response, and additional intradermal testing of that specific antigen is not conducted. In the circumstance of a 9 mm or larger wheal, the patient is assigned an IDT EP of No. 6 for that antigen, and testing for that antigen is complete. In testing antigens that demonstrate wheals of less than 9 mmby prick testing, responses are categorized as those less than 3 mm in diameter and those 3 mmto 8 mmin diameter. Patients in the first category are classified as negative to that antigen on the basis of prick testing alone. To further assess whether the patient is able to respond to a less concentrated antigen, and is therefore truly positive to that antigen, a No. 2 IDT dilution [1 : 500 weight per volume (w/v)] of that antigen is then placed intradermally in the lateral aspect of the upper arm. A period of 10 minutes is allowed to elapse, and the diameter of that intradermal wheal is measured. A wheal of 7 mm or greater in diameter is interpreted as positive, and the patient is therefore considered to be reactive to that antigen. To be conservative and safe, the endpoint value assigned to that positive test is IDT EP 3. If the wheal size is less than 7 mm, the patient is judged to be negative to that antigen. In the second category, where the prick test demonstrates a wheal size of between 3 and 8 mm in diameter, testing has already classified the patient as positive to that antigen. A single intradermal test of IDT No. 5 (1 : 62,500 w/v) is then used to further refine the degree of positivity to that antigen. The purpose of this intradermal test is to assess whether the prick test reflects a maximal antigen challenge, or whether placement of a less concentrated challenge would also produce an effect. Once the No. 5 wheal is placed, 10 minutes is allowed to elapse and the size of the wheal is measured. Three results may be encountered after placement and measurement of this wheal: a wheal of less than 7 mm in diameter is interpreted as a negative response, and a value of IDT EP 4 is assigned; a wheal of 7 or 8 mm in diameter is interpreted as a positive test, and a value of IDT EP 5 is assigned; a wheal of 9 mm or greater in diameter is a maximal response to the prick test, and a value of IDT EP 6 is assigned. The purpose of the blended MQT approach to testing is to provide a quantitative value for antigen sensitivity that can be used for preparation of immunotherapy. To allow ready communication of testing results, and to employ a system that has acceptance and familiarity, MQT testing defines degree of sensitization on the basis of IDT endpoints. Through using IDT methodology in this manner, the preparation of vials for immunotherapy can proceed in a standard fashion. The blend of skin responses to the prick test and the subsequent single intradermal test allows the assignment of discrete IDT endpoints, as noted in Fig. 7-1. Once again, the initial prick test allows a determination of prick-negative (<3 mm), prick-positive (3 mm or greater), and maximum positivity (9 mm or greater). A negative prick test is refined with a No. 2 dilution intradermal test, and it is either intradermal-negative (<7 mm) or intradermal positive (7 mm or greater). A No. 2 intradermal-negative test is interpreted as negative, and a No. 2 intradermal-positive test is assigned a value of IDT EP 3. A positive prick test is refined with a No. 5 dilution intradermal test, and it is either intradermal-negative (<7mm), intradermal-positive (7 mm or 8 mm), or maximally positive (9 mm or greater). A No. 5 intradermal-negative test is assigned a value of IDT EP 4, a No. 5 intradermal-positive test is assigned a value of IDT EP 5, and a maximally positive No. 5 intradermal test is assigned a value of IDT EP 6. In the case of a maximally positive prick test, again this test is assigned a value of IDT EP 6. The endpoints determined through MQTare interpreted in the same way as they would be for full IDT testing, and vial preparation proceeds in the same manner. In using blended techniques, it is useful to employ vial testing to confirm the safety of each treatment vial prior to proceeding with injections. The method of vial testing and its interpretation is described in Chapter 8. The principles of quantitative testing have been employed in skin testing for inhalant allergy for many years. They have provided a sound foundation for the detection of clinical allergy, and they have assisted in determining the degree of reactivity to specific antigens. These principles have a long record of safety and have been used effectively by thousands of physicians to manage immunotherapy. MQT provides an efficient method for evaluating allergic responsiveness and allows accurate assessments of quantitative sensitivity in an efficient and cost-effective manner. It offers the practicing allergist an additional tool that may be employed for testing and treatment. The effective allergist must have a variety of tools and techniques available for practice, and the flexible application of these methods permits successful diagnosis and management of the allergic patient.
Blending Methods: Modified Quantitative Testing (MQT)
John H. Krouse
♦ The Technique of MQT
♦ Interpretation of MQT Results
♦ Conclusion
< div class='tao-gold-member'>
Blending Methods: Modified Quantitative Testing (MQT)
Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree