Fig. 4.1
T1-weighted sagittal MRI sequence (a) showing nonspecific thickening of synovium with tunnel widening possibly consistent with graft immune rejection. (b) An inflammatory medial parapatellar plica is noted on axial images
Midterm
Tunnel Placement
Tunnel malposition is the most common recognized technical error during ACL reconstruction, accounting for 24–80 % of failures [2, 23]. Improperly placed tunnels may lead to poor graft kinematics, impingement, and ultimate failure of ACL reconstruction [24]. This has led to expanding interest in anatomic ACL reconstruction. Although either tunnel may be malpositioned, the femoral tunnel has historically comprised the majority of placement errors [25, 26]. Indeed, one of the most common etiologies of recurrent instability identified after ACL reconstruction is the central (vertical) placement of the femoral tunnel high in the intercondylar notch. This has been speculated to be due to the fact that the femoral tunnel had been dictated by the tibial tunnel in transtibial reconstruction techniques [27, 28]. Studies have shown that the transtibial positioning tends to lead to a high anteromedial and nonanatomic femoral tunnel position [29–31]. Anterior placement of femoral tunnels may lead to excessive strain in both flexion (when tensioned in extension) and extension (when tensioned in flexion). Vertical grafts that result from placing the femoral tunnel too central in the intercondylar notch do not constrain the knee under rotatory forces, so while anteroposterior stability may be intact, rotational stability is not (Fig. 4.2a, b). Several cadaveric studies have shown that there is improved stability, particularly in response to combined anterolateral rotatory loads, when the femoral tunnel is moved from a position on the “roof” of the intercondylar notch to a more anatomic position “down the wall” of the notch [32, 33].
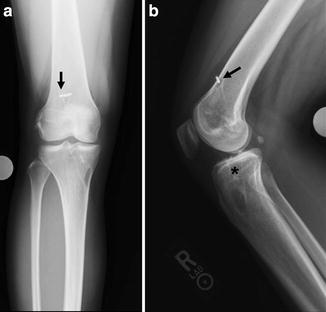

Fig. 4.2
AP (a) and lateral (b) radiographs reveal vertical graft orientation with suspensory fixation seen anteriorly and central in the femur (arrows). The tibial tunnel is placed posteriorly (star)
Another common error is a posterior tibial tunnel leading to a vertical femoral tunnel, which came about early in the history of transtibial techniques. With newer techniques such as using an anteromedial portal for femoral tunnel placement and thus independent placement of the tibial tunnel, excessive anterior placement of the tibial tunnel can be problematic. A cadaveric study using computer navigation and a mechanized Lachman and pivot shift showed that while keeping the femoral tunnel constant, placing the tibial tunnel further anterior on the tibial plateau afforded better control over the Lachman and pivot shift tests, but increased risk of impingement. It is therefore important to balance the risk of instability and impingement by placing the tibial tunnel in an anatomic position. Even with anatomic graft placement however, graft failure has been reported in 13 % of ACLR over the first 30 months postoperatively [1]. It is therefore important to understand that factors other than tunnel malposition can cause midterm ACL graft failure.
Impingement
As previously mentioned, it has been shown that anterior placement of the tibial tunnel improves knee stability in both the A-P and rotatory planes [34]; however, anterior tibial tunnels also lead to greater graft impingement in extension [35, 36] and excessive strain in knee flexion. It is important to recognize that while anterior placement of the tibial tunnel improves sagittal graft obliquity and improves stability and kinematics, excessive anteriorization of the tibial tunnel will lead to impingement and the resultant inability to extend the knee [34]. Graft erosion may also occur from tibial tunnel placement too medially or laterally, resulting from impingement with the intercondylar notch wall or PCL [37].
Nonanatomic placement of femoral tunnels may also lead to graft impingement. A cadaveric study was performed using computer navigation to evaluate femoral tunnel position on graft impingement [38]. Varying femoral tunnel positioning from AM to center to PL footprints resulted in significantly decreasing angles of impingement in extension, indicating that more anteriorly placed femoral tunnels are more prone to impingement in extension. Indeed tunnel positioning in the central region of the femoral and tibia footprint may be the most effective means of controlling stability and avoiding impingement in the ACL reconstruction.
Graft Elongation
For a successful reconstruction to maintain stability after leaving the operating room, graft tension must be maintained and protected. Instability in the absence of other causes of graft failure such as fixation failure, biological failure, tunnel malposition, or trauma should alert the surgeon to the possibility of graft elongation. Graft elongation, or creep, is defined as elongation due to non-recoverable stretch and loss of stiffness and may lead to gradual failure [39]. Greater graft elongation and creep may be due to decreased time of preconditioning grafts and inappropriate cycling of the graft prior to fixation. In vitro studies suggest that preconditioning grafts with a constant tensile load may be beneficial in preventing in vivo elongation [40]. In addition, overly aggressive early postoperative physical therapy prior to graft healing may also hinder incorporation or promote creep [41]. This occurs by graft motion and/or pullout. Soft tissue grafts have been shown to have a higher rate of failure due to creep when compared to BTB [42, 43] (Fig. 4.3).
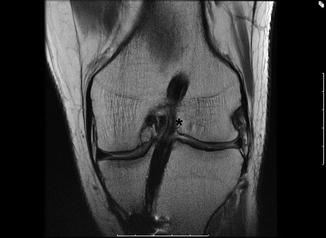

Fig. 4.3
Coronal T1-weighted MRI sequence showing a vertical soft tissue graft resulting from a centrally placed femoral tunnel. Graft laxity and buckling are seen with nonlinear graft fibers (star)
Secondary Stabilizers
It is important to recognize and appropriately address all laxity in the secondary stabilizers of the knee to avoid excessive tensile forces and eventual ACL graft failure. Unrecognized associated ligamentous injury at the time of primary reconstruction accounts for around 15 % of ACL failures. With deficient secondary anterior stabilizers, the ACL graft will provide restraint for the first 6 months but increased demand activities will lead to a gradual recurrence of instability [44]. In a classic paper, O’Brien [45] reported on 80 primary ACL reconstructions and found that all of those with postoperative clinical instability had evidence of associated ligamentous instability. The most commonly unrecognized instability is posterolateral corner injury, followed by posteromedial injury and medial meniscus [45–48].
Injury to the posterolateral corner in an ACL-deficient knee results in increased instability. In a cadaveric study [49], the anterior tibial translation and anterolateral rotational instability of the knee were significantly increased after sectioning the ACL (p < 0.05). Sectioning of the lateral collateral ligament further increased the anterolateral rotational instability. Subsequently sectioning of the popliteus complex increased the anterior tibial translation (p < 0.05) but not the anterolateral rotational instability.
As with the lateral sides, the posteromedial aspect of the knee, which consists of the superficial and deep medial collateral ligament (MCL) and posterior oblique ligament, contributes to anterior knee stability [50]. Combined MCL and ACL deficiency were found to significantly increase anterior tibial translation relative to the ACL-deficient knee at flexion angles above 60°. The MCL is thus an important contributor to anterior stability at higher knee flexion angles. These associated ligamentous and meniscal injuries are important to recognize and treat appropriately to prevent ACL graft failure.
In addition to the lateral and medial side, medial meniscal deficiency is another common associated injury pattern. Ahn [51] reported on knee kinematics with ACL deficiency and medial meniscal injury and repair. Medial meniscal tear resulted in increased anterior-posterior tibial translation at all flexion angles except 90° (p < 0.05) and the repaired medial meniscus showed a decreased anterior-posterior translation at all flexion angles except 60° compared to the torn meniscal state. This signifies the importance of recognizing and treating associated medial meniscal pathology. While the medial meniscus and status of the medial compartment is a crucial component of knee stability, the development and standardization of the mechanized pivot shift has enabled researchers to evaluate the rotational stability of cadaveric knees for biomechanical studies, rather than just A-P stability. In a cadaveric study using a Lachman and mechanized pivot shift, Ahn’s results were confirmed in that the medial meniscus was a critical secondary stabilizer during Lachman examination, but the lateral meniscus was a more important restraint to rotatory loads during a pivoting maneuver [52]. In addition to secondary restraint, meniscectomized knees may lead to cartilage loss and resultant malalignment. In the setting of a meniscectomized knee with cartilage loss and malalignment, concomitant bony realignment procedures are required with ACL revision surgery (Fig. 4.4a, b).
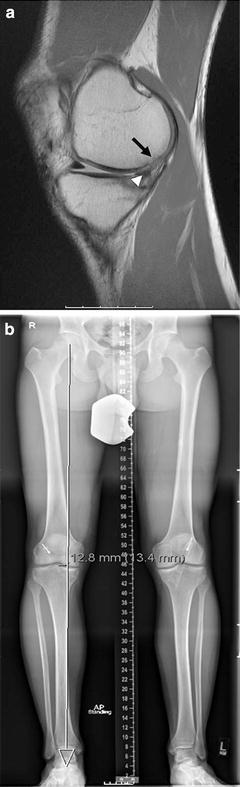

Fig. 4.4
T1-weighted sagittal MRI sequence (a) showing posterior chondral wear (black arrow) effects of prior meniscectomy (white arrowhead), and anterior tibial translation of a failed ACL reconstruction. Hip-to-ankle alignment radiographs (b) indicate right knee varus malalignment
Late
Trauma
Knee trauma following ACL reconstruction may be a devastating and unpredictable cause of graft failure, and accounts for 32–70 % of revision cases [2, 17]. Traumatic mechanism and force may be similar to the original injury with an audible “pop,” laxity, hemarthrosis, and inability to walk, or may be from an event in which there was much less force than the original injury. Because of this, patients may present reporting only minor recent trauma; however, new onset instability or laxity after a period of functional stability indicates potential traumatic failure. Therefore minor trauma with subjective complaints of instability should not be ignored. Traumatic failures may occur early during graft incorporation from overaggressive physical therapy or premature return to athletic activity, or later after the graft has matured and the patient has returned to athletic activity (typically >1 year postoperatively). Once the graft has matured, the risk of reinjury is similar to that of the contralateral ACL. Early aggressive rehabilitation and premature return to sport may lead to reinjury. Postoperative bracing is a common practice with the goal of preventing reinjury. Some believe that there is an improvement in knee extension, decreased pain and graft strain, and protection from excessive force. A systematic review of 12 randomized controlled trials (RCTs) failed to show evidence that pain, knee range of motion, graft stability, or protection from subsequent reinjury was affected by brace use [53].
Conclusion
The purpose of this chapter was to review the biomechanics of graft failure. Most literature on this topic relates to technique-related issues in ACL reconstruction. Indeed, the most widely cited and well-studied reason for graft failure is tunnel malposition [54] and a large body of literature is devoted to the concept of “anatomic” placement of graft tunnels. In spite of this focus on refining tunnel position, graft failure is still found in up to 13 % of anatomic ACL reconstruction [1]. This suggests that while surgical technique is important, other factors that may be patient-specific (and minimally affected by surgical technique) may also have a role in graft failure. Indeed, in a recent meta-analysis of patients undergoing ACL reconstruction, the rerupture rate of the ACL graft was 6 % while the rupture rate of the contralateral, native ACL in these patients was 12 % [55]. The fact that patients who undergo an ACL reconstruction have twice the risk of tearing their contralateral ACL as their graft suggests that patient-related factors such as genetics, gender, alignment, bony morphology, and landing mechanics may have dominant roles in the etiology of failure after ACL surgery.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








