Chapter 18 Biologic Mesh Choices for Surgical Repair
1 Indications for the Use of Biologic Mesh Materials
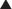 The major indication for the use of biologic meshes during abdominal wall surgery is improved wound healing. This most often translates into the minimization of wound complications. Studies have reported wound infection rates of 4% to 16% after incisional ventral hernia repair. The application of synthetic mesh to reinforce abdominal wall repairs significantly reduced recurrence rates, but it introduced an increased risk of complicated wound infections, synthetic mesh infections and erosion into the bowel. Level one data find that recurrence rate and wound infection are related, in that a postoperative wound infection significantly increases the incidence of recurrent incisional hernia. Another inherent limitation with synthetic meshes is shrinkage. It is well measured now in animal models and with human explants that synthetic meshes lose on the average 20% of their surface area following implantation. This is believed to contribute to high hernia recurrence rates. The era of managing traumatized patients with open abdomens expanded the use of biologic mesh based abdominal wall reconstruction. Here there was concern for wound contamination and exposure of synthetic prostheses to the abdominal viscera. Tenuous or insufficient skin coverage also is suggested as an indication for the use of a biologic mesh during abdominal wall repair.
The major indication for the use of biologic meshes during abdominal wall surgery is improved wound healing. This most often translates into the minimization of wound complications. Studies have reported wound infection rates of 4% to 16% after incisional ventral hernia repair. The application of synthetic mesh to reinforce abdominal wall repairs significantly reduced recurrence rates, but it introduced an increased risk of complicated wound infections, synthetic mesh infections and erosion into the bowel. Level one data find that recurrence rate and wound infection are related, in that a postoperative wound infection significantly increases the incidence of recurrent incisional hernia. Another inherent limitation with synthetic meshes is shrinkage. It is well measured now in animal models and with human explants that synthetic meshes lose on the average 20% of their surface area following implantation. This is believed to contribute to high hernia recurrence rates. The era of managing traumatized patients with open abdomens expanded the use of biologic mesh based abdominal wall reconstruction. Here there was concern for wound contamination and exposure of synthetic prostheses to the abdominal viscera. Tenuous or insufficient skin coverage also is suggested as an indication for the use of a biologic mesh during abdominal wall repair. The risk of laparotomy wound complications may be graded and wound outcomes predicted based on patient comorbidities and level of contamination. Now long-term experience with synthetic meshes in the abdominal wall raises awareness and concern for acute and chronic infections, as well as intestinal injury. The growing problem of infected mesh explantation also drove increased use of biologic meshes as replacements. Obesity is now a measurable risk factor for increased wound complications following ventral hernia repair.
The risk of laparotomy wound complications may be graded and wound outcomes predicted based on patient comorbidities and level of contamination. Now long-term experience with synthetic meshes in the abdominal wall raises awareness and concern for acute and chronic infections, as well as intestinal injury. The growing problem of infected mesh explantation also drove increased use of biologic meshes as replacements. Obesity is now a measurable risk factor for increased wound complications following ventral hernia repair. The growing popularity of major reconstructive procedures for large ventral hernias also expanded the use of biologic meshes. The perceived need for reinforcement following component separation procedures and the concern for the implantation of synthetic materials into these large surface area wounds led to this adaptation of biologic mesh. Clinical studies have supported reduced recurrence rates using mesh reinforcement during component separation.
The growing popularity of major reconstructive procedures for large ventral hernias also expanded the use of biologic meshes. The perceived need for reinforcement following component separation procedures and the concern for the implantation of synthetic materials into these large surface area wounds led to this adaptation of biologic mesh. Clinical studies have supported reduced recurrence rates using mesh reinforcement during component separation.2 Tissue Sources for Biologic Mesh Materials (Table 18-1)
 The application of biologic materials to abdominal wall repair began with autologous source material. Tensor fascia lata (TFL) has the widest experience. De-epithelialized autologous dermis also has been used. Muscle flaps and now even composite tissue transfers, both pedicle based and free flaps, have been described. Autologous small bowel and omentum also are reported as tissue sources for abdominal wall repair. Donor site morbidity is the obvious limitation of autologous biologic tissue sources. There are no large studies of TFL or autologous dermis in abdominal wall repair, and the long-term durability, especially when used as bridging repair, is not known.
The application of biologic materials to abdominal wall repair began with autologous source material. Tensor fascia lata (TFL) has the widest experience. De-epithelialized autologous dermis also has been used. Muscle flaps and now even composite tissue transfers, both pedicle based and free flaps, have been described. Autologous small bowel and omentum also are reported as tissue sources for abdominal wall repair. Donor site morbidity is the obvious limitation of autologous biologic tissue sources. There are no large studies of TFL or autologous dermis in abdominal wall repair, and the long-term durability, especially when used as bridging repair, is not known. The need for more readily available biologic reconstructive matrices and the limitation of autologous tissue donor site morbidity led to the development of off-the-shelf human allograft sources for abdominal wall reconstruction. To date, this has been dominated by human dermal allografts. Although early results have been good, there is an inherent limitation in the need for human tissue banking and the risk of transmission of infectious disease. Before processing, potential donors undergo screening that includes a medical and social history review, physical examination and serologic testing to minimize the risk of disease transmission. Donor tissue that passes rigorous screening then undergoes physical and chemical processing to further reduce the risk of disease transmission before implantation. Occasionally, social and cultural restrictions may preclude the application of human tissue sources to specific patient groups.
The need for more readily available biologic reconstructive matrices and the limitation of autologous tissue donor site morbidity led to the development of off-the-shelf human allograft sources for abdominal wall reconstruction. To date, this has been dominated by human dermal allografts. Although early results have been good, there is an inherent limitation in the need for human tissue banking and the risk of transmission of infectious disease. Before processing, potential donors undergo screening that includes a medical and social history review, physical examination and serologic testing to minimize the risk of disease transmission. Donor tissue that passes rigorous screening then undergoes physical and chemical processing to further reduce the risk of disease transmission before implantation. Occasionally, social and cultural restrictions may preclude the application of human tissue sources to specific patient groups. The unique regulatory limitations of human tissue banking and the need for better processing quality control drove the introduction of xenografts as biologic materials for abdominal wall reconstruction. This also reduces the cost of xenografts that on the average are 50% less expensive than allografts. This trend has been dominated by porcine intestinal submucosa, porcine dermis, bovine fetal dermis, and bovine adult pericardium. One fundamental limitation of xenografts is the presence in humans of preformed anti-xenograft antibodies. The anti-galactose-alpha-1,3-galactose antibodies are the best described. There is also evidence for the induction of nonspecific inflammatory pathways as part of a generalized foreign body reaction. With xenografts as with allografts, social and cultural restrictions may apply.
The unique regulatory limitations of human tissue banking and the need for better processing quality control drove the introduction of xenografts as biologic materials for abdominal wall reconstruction. This also reduces the cost of xenografts that on the average are 50% less expensive than allografts. This trend has been dominated by porcine intestinal submucosa, porcine dermis, bovine fetal dermis, and bovine adult pericardium. One fundamental limitation of xenografts is the presence in humans of preformed anti-xenograft antibodies. The anti-galactose-alpha-1,3-galactose antibodies are the best described. There is also evidence for the induction of nonspecific inflammatory pathways as part of a generalized foreign body reaction. With xenografts as with allografts, social and cultural restrictions may apply.3 Biologic Mesh Modification and Processing
 The use of biologic allografts and xenografts for soft tissue repair required the development of processing techniques that allowed for the removal of immunogenic cells, while preserving a functional biologic matrix. This remnant biologic matrix is constructed primarily of collagen, and depending on the degree of preservation of the native structure, maintains measurable biologic function even in the acellularized state.
The use of biologic allografts and xenografts for soft tissue repair required the development of processing techniques that allowed for the removal of immunogenic cells, while preserving a functional biologic matrix. This remnant biologic matrix is constructed primarily of collagen, and depending on the degree of preservation of the native structure, maintains measurable biologic function even in the acellularized state.Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree