Clinical criteria
Pathological criteria
Cyclical nodularity
Nonproliferative lesions
Mastalgia
Cysts
Nodule (localised, cyclic)
Mammary duct ectasia
Fibroadenoma
Other nonproliferative lesions
Galactocele
Proliferative lesions without atypia
Cyst
Adenosis and Fibrocystic changes
Secretion
Fibroepithelial (fibroadenoma) and related lesionsa
Galactorrhea
Papilloma and benign papillary lesions
Abnormal secretion
Myoepithelial lesions
Infections
Sclerosing lesions (sclerosing adenosis, radial scar)
Milk stasis
Proliferative lesions with atypia
Lactating mastitis
Atypical ductal hyperplasia (ADH) also known as DIN 1b
Non-lactating mastitis:
Atypical lobular hyperplasia (ALH) also known as LIN 1
Periductal mastitis
Flat epithelial atypia also known as DIN 1a
Complicated cysts
Lobular carcinoma in situ (LCIS)b also known as LIN 2
Extramammary infections
As shown in Fig. 9.1, nearly all breast pathology originates in the terminal ductal lobular unit (TDLU), considered the functional unit of the breast and the most actively proliferating part. So that, for instance, cysts may be the consequence of the unfolding of the terminal ducts and lobular units, adenosis is a lobulocentric proliferation of ductules with epithelial and myoepithelial cells, fibroadenoma arises from the epithelium and intralobular stroma, myoepithelioma has a tubular structure surrounded by myoepithelial cells, and so on. Indeed, the only common lesion believed to be strictly of ductal origin may be the larger solitary intraductal papilloma.
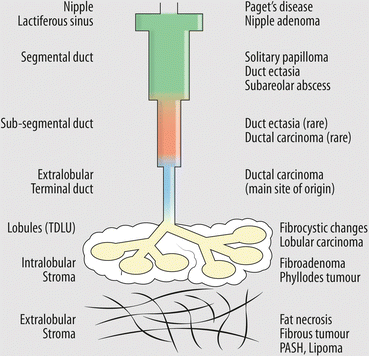

Fig. 9.1
Schematic drawing illustrates the site of origin (on the left) for the most common breast diseases (on the right)
ANDI CLASSIFICATION. Aberrations of normal development and involution (ANDI) classification is designed to embrace all aspects of benign disorders of the breast, starting from breast development and physiology to clinical symptoms and signs, up to histological patterns [2]. New sound concepts of pathogenesis clearly show that:
Benign breast conditions are practically a universal phenomenon among women.
Benign pathological states account for approximately 90 % of the clinical presentations related to the breast, and these diseases are more common in females 30–50 years old; thus, they are hormonal in nature.
Previously there was a tendency to include almost all benign breast disorders and pathology under the designation of fibrocystic disease or mammary dysplasia, but today these terms are unsuitable.
The term fibrocystic disease applied to a biopsy or a palpable breast mass is nonspecific and often includes normal physiologic and morphologic changes in the breast along with specific benign disease processes.
Almost all benign breast disorders simply are relatively minor aberrations of the normal processes of development, cyclical hormonal response and involution.
On account of these principles, the ANDI classification, asserting that most benign disorders are related to normal processes of reproductive life, replaces the conventional view between ‘normal’ and ‘disease’. Since there is a spectrum ranging from normal through slight abnormality (aberration) and occasionally to disease, the definition of normal and abnormal should be considered pragmatic (Table 9.2). Therefore, the purpose of the term ANDI is to prevent the use of the word disease for normal changes and to eliminate confusion.
Table 9.2
Benign changes of the breast according to clinical and pathological criteria, in the ANDI (aberration) and non-ANDI (disease) classification [2]
Stage | Normal process | Aberration | Disease |
|---|---|---|---|
Early reproductive (15–25 years) | Lobular development | Fibroadenoma | Giant fibroadenoma |
Stromal development | Adolescent hypertrophy | Gigantomastia | |
Nipple eversion | Nipple inversion | Subareolar abscess/mammary duct fistula | |
Mature reproductive (25–40 years) | Cyclical changes of menstruation | Cyclical mastalgia | Severe mastalgia |
Nodularity | |||
Epithelial hyperplasia of pregnancy | Blood nipple discharge | ||
Involution (35–55 years) | Lobular involution | Macrocysts Sclerosing lesions | |
Duct involution Dilatation Sclerosis | Duct ectasia Nipple retraction | Periductal mastitis/abscess | |
Epithelial turnover | Simple epithelial hyperplasia | Epithelial hyperplasia with atypia | |
Non-ANDI | Condition of well-defined extramammary aetiology, such as fat necrosis or lactational abscess, together with extrinsic precipitating factors such as trauma, infection, smoking, etc. | ||
The ANDI classification deserves to be reported, as it is most helpful in understanding pathological process and in guiding rational clinical management. Nonetheless, the currently used grouping based on proliferative/nonproliferative changes with/without atypia is preferable but not essential.
9.2 Nonproliferative Benign Breast Lesions
Clinical Practice Points
Macrocysts represent the most common palpable benign breast mass in premenopausal women.
The common subset of simple cyst is very easy to detect, but other subsets of cyst may be difficult to diagnose as benign.
Fibrocystic changes are not a disease as such, but instead a general term that refers to a group of anomalies, symptoms and conditions that form part of the spectrum of benign breast pathology.
Minor nonproliferative lesions do not cause palpable mass but are occasional findings on pathological samples or on mammogram.
9.2.1 Cyst of the Breast
Cysts are the result of a period of fluctuating involution of lobules extending over 20 years. The exact mechanism is not well understood, but it appears that the normal epithelial involution of the lobule is dependent – and not always integrated – on the continuing presence of the stroma around it. If the stroma disappears too early, the epithelial acini remain and may form microcysts, setting the pattern for macrocyst development by obstruction of the efferent ductules.
Some descriptions still do not provide adequate clarity and consistency. Also the fact that macrocysts appear to develop in two directions – apocrine and non-apocrine cysts – is something which is as yet poorly understood, but it is evident both develop from a common origin of microcystic involution.
Macrocysts. They constitute the most common discrete benign breast mass, estimated to occur in 7–10 % of all women. Simple cysts are fluid filled, round or ovoid masses derived (but not always) from apocrine metaplasia of the terminal duct lobular unit (TLDU). They are common in the premenopausal women between 35 and 50 years old and in women who take hormone replacement therapy at any age.
Microcyst. Asymptomatic microcysts are often found mainly in ultrasound scanning at any age, but mostly in the last decade of reproductive life.
Cysts are clinically significant for many reasons. As macrocysts cause considerable anxiety, mainly for the localised pain due to sudden onset in acute enlargement. Moreover they present as lumps in women of an age where BC is more likely to occur, so they are assumed to be cancers when first discovered.
Furthermore, there is some evidence that recurrent macrocysts may increase the risk of BC slightly, though opinions about, and evidence for, the details of the associated cancer risk are not uniform. Actually macrocysts fall into two comprehensive groups: those with a persisting apocrine cell lining and active secretion/concentration of many substances and those lined by flattened cells and metabolically much less active. In any case cysts may cause diagnostic confusion and psychological consequences, mainly when they are often recurrent and bilateral, requiring several visits to the outpatient clinic for assessment.
Finally, there are many kinds of cysts, and, because BCs only rarely present as a cystic appearance, some of them could be due to intracystic papillary tumours of benign histology or low-grade malignancy.
Cysts present as a lump in the breast that is normally smooth and fluctuant on clinical examination. If it is in tension or fast recurrent, simple macrocyst may be adequately treated by aspiration (Fig. 9.2). Upon aspiration the fluid within a breast cyst is normally either clear or turbid and can be any colour from pale to black, while no blood is seen.
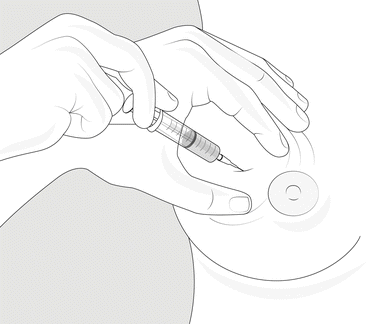

Fig. 9.2
Fine needle aspiration of a cyst. The removal of cyst fluid by fine needle aspiration is somewhat therapeutic if there is no residual mass after aspiration and no recurrence in approximately 3 months
The amount of fluid varies, generally between 2 and 10 ml, but can be considerably more. Usually the mass disappears after aspiration leaving a temporary defect. Afterwards the breast is re-examined, and if no further palpable abnormality is felt and if there is nothing on ultrasound to cause concern, the diagnosis is confirmed and no more steps need be done. Patients are reassured and warned that the cyst may recur or that they may develop further cysts in the same or other breast.
Since cystic lesions are furthermost common, true breast cysts should be classified as simple, complicated or complex based upon the characteristics identified by ultrasound evaluation. Moreover it is worth to remember some cysts may be secondary to a trauma or infection or associated with proliferative (as papilloma or carcinoma) or necrotic conditions, or may be false cyst (Table 9.3).
Table 9.3
Primary and secondary cysts of the breast
True breast cysts | Simple cyst |
Typical ultrasonic features: well circumscribed, no internal echoes, posterior acoustic enhancement, no vascular flow | |
Subsets are: | |
Clustered microcysts | |
Cyst with thin septa | |
Juvenile cyst | |
Complicated cyst | |
Low-level internal echoes, thick wall or internal septa, no solid components, no vascular flow | |
Complex cyst | |
Thick wall and/or septa more than 0.5 mm, presence of cystic and solid components, no posterior acoustic enhancement | |
Secondary cysts | Galactocele |
Oil cyst of fat necrosis | |
Abscess associated with periductal mastitis | |
Liquefied haematoma | |
Postsurgical fluid collection | |
Cysts associated with proliferative or necrotic conditions | Papillary tumour |
Papillary carcinoma | |
Phyllodes tumour | |
Necrotic carcinoma | |
False cysts | Epidermal cyst |
Parasitic cyst |
Simple cyst. It is well circumscribed with ultrasonic features that include posterior acoustic enhancement, without internal echoes (anechoic), solid components or Doppler signal. Subsets of simple cysts are:
Clustered microcysts – a cluster of simple anechoic cysts, each smaller than 2–3 mm, without discrete solid components
Cysts with thin septa that are less than 0.5 mm in thickness
‘Juvenile’ cyst – a clinical term attributed to cyst in young girls up to the age of 20
Complicated cyst. It is defined by ultrasound criteria as a mass with homogeneous low-level internal echoes due to echogenic debris; without solid components, thick walls or thick septa; and without vascular flow. Some cysts will refill after 2–3 aspirations because of their thick walls, but a suspicion of intraluminal proliferation should be considered.
Complex cyst. It is defined by ultrasound criteria as a mass with thick walls and/or septa greater than 0.5 mm; presence of cystic and solid components, internal echoes, fluid debris level, irregular borders and septation; and absence of posterior wall enhancement. The ultrasound appearance of complex cysts can demonstrate anechoic and echogenic components. Needle aspiration can show atypical fluids (bloody or purulent). All complex lesions should be investigated.
Workup of cystic masses. Ultrasound is beneficial for showing the cystic nature of these lesions and even more helpful with poorly defined or complex cysts. It is also valuable to ensure complete emptying of recurrent cysts in order to exclude the presence of intraluminal masses. It should be considered anyhow that leakage from a cyst gives causes surrounding inflammation with altered ultrasound appearances and may leave a residual mass after aspiration [3].
Mammogram usually detects cysts only if size is sufficient and breast density allows it. If indicated, mammogram should be performed before aspiration since image usually appears normal after aspiration, but only if the aspiration has been not traumatic.
Only a bloody aspirate needs to be investigated, whereas normal cystic fluid could be usually discarded. Cysts yielding blood-stained fluid should be submitted for cytological evaluation and in any case carefully followed even if triple assessment is negative. Surgical excision should be considered for any suspicious lesion, when multiple recurrences need repeated aspirations and when the patient refuses such repeated manoeuvres.
When blood is aspirated from a cyst (even if the cyst totally disappears) or when there is a residual palpable abnormality, a histological sampling is needed, though a period of observation for a short time may be justified in certain cases, depending on the results of mammography and cytopathology. Under these circumstances, a delayed diagnosis of a cancer mistaken for a cyst can be a serious cause of legal litigation.
9.2.2 Mammary Duct Ectasia and Partly Related Conditions
OVERVIEW. Mammary duct ectasia is basically a dilatation of the main ducts more or less associated with stasis of the secretions and ensuing reactive, inflammatory or infectious conditions. These various processes, individual but interrelated, explain the protean clinical presentations of the so-called mammary duct ectasia/periductal complex.
There are four main theories that try to explain duct dilatation:
A progressive failure of the muscle wall of the duct, perhaps due to the relaxation effect of progesterone.
A failure of absorption of the duct secretion due to inadequate lymphatic flow.
An obstructive phenomenon due to blockage of the ducts at their termination by squamous cell debris, with leakage of highly irritant lipid material into the periductal tissue.
A periductal inflammation as the primary process, perhaps a form of autoimmune disease, with muscle damage and duct dilatation as secondary phenomena. Obviously this theory contrasts sharply with the others.
Duct ectasia may indeed be involved in a number of processes that may exist alone or in combination with it. Some are subclinical minor variants of normality, as physiological involution, while others have a spectrum of clinical and pathological manifestations, which extends to disease with severe morbidity. A large spectrum of conditions is possible, where much confusion still arises due to failure to differentiate between histological findings and clinical disease entities.
In the attempt to include the clinical manifestations within a single all-embracing disease process, definitions as duct ectasia/periductal mastitis (DE/PM) complex and mammary duct–associated inflammatory disease sequence (MDAIDS) have been proposed. Actually all approaches are incompatible with the breadth of clinical manifestations or the observed pathology, and, furthermore, all conditions could also be regarded as one aspect of fibrocystic changes.
Mammary duct ectasia-related conditions present clinically in many ways, at times giving rise to all common breast symptoms (nipple discharge, pain, mass), as well as to other less common manifestations including inflammation, abscess, fistula, chronic persistent mastalgia and nipple inversion or sometimes retraction (for this distinction, see Sect. 11.1.1). As told before, all conditions may present by themselves or in combination with others. Afore dealing with the different phases of the pathogenesis connecting them to each other, it is useful set them out separately, as in Table 9.4.
Table 9.4
The clinical spectrum of presentations of minor reactive duct ectasia to duct ectasia associated with inflammatory diseases
Disorders/process | Influential factors | Main clinical symptoms | Frequency |
|---|---|---|---|
Duct ectasia regressive | Constitutional (fatty breasts, congenital nipple inversion, etc.) | Asymptomatic | By the age of 70, 40 % of women have substantial, bilateral and almost never symptomatic duct ectasia |
Hormonal (unclear action) | Nipple discharge: only elicited, coloured, thick, creamy, sometimes bloody | ||
Periductal fibrosis reactive or mild inflammatory | Duct obstruction with stagnant fluids | Asymptomatic or poorly symptomatic | Not common, sometimes unilateral |
Nipple inversion | Nipple inversion, more noticeable if pre-existing | ||
Paraesthesias | |||
Periductal mastitis inflammatory | Environmental (as smoking nicotine) | Pain (usually noncyclical mastalgia) | Rare, more common in young women (mean age 35 years) |
Other reported elements (as nutritional) not fully proven | Subareolar inflammatory mass (common) | Two–three times more common in smokers as compared to age-matched control subjects | |
Nipple inversion (rarely, retraction) | |||
Subareolar breast abscess infectious | (see Sect. 8.3) | Abscess that usually drains spontaneously | About 20–30 % of patients with severe periductal mastitis |
Mixed flora (aerobes and anaerobes) usually typical of (and probably coming from) those in the mouth and vagina | |||
Periareolar fistula complicated | (see Sect. 8.3) | Sinus opening to fistula | About 20 % of patients with subareolar breast abscess |
Inappropriate or inadequate surgery of subareolar abscess | Recurrence with repeated discharge via fistula | ||
Secondary conditions associated | Persistent nipple discharge | Crusting of the nipple | Very rare, except crusting |
Stasis of secretions | Dermatitis or eczema of the areola | ||
Inflammatory chronic diseases (only in part) |
Duct ectasia is a regressive, involutional process, a normal breast change. As women reach menopause and the breasts age, the major ducts get shorter and wider. By the age of 70, 40 % of women have substantial, bilateral and only slightly symptomatic duct ectasia.
Periductal fibrosis is a reactive or mild inflammatory condition, less common, usually without symptoms and occasionally found on biopsies done for another problem. Accumulation of detritus in the widened duct lumen can cause a fibrous thickening of the many elastic fibres of the wall.
Periductal mastitis is an inflammatory process, usually due to fibrous obliteration of the ducts, reported under many terms (comedomastitis, mastitis obliterans, etc.). Periductal mastitis can affect women of all ages, but most cases occur in premenopausal women (mean age about 35 years), two to three times more common in smokers as compared to age-matched control. These data suggest periductal mastitis and duct ectasia are separate conditions which affect different age groups, have different aetiologies and should be considered as separate entities. In few cases it can be accepted as part of the normal involution which may lead to the otherwise asymptomatic nipple retraction in the older woman.
Periductal mastitis is usually symptomatic. The breast becomes tender and hot to touch, and the skin may appear reddened. In almost all cases a subareolar inflammatory mass is palpable. Pain (usually noncyclical mastalgia) is common too, also in the absence of other symptoms. Nipple retraction, inversion or discharges are present in approximately 20 % of patients.
Subareolar breast abscess is an infectious nonreversible process, which occurs in about one out of five patients with periductal mastitis, but can also occur without previous symptoms. Purulent collection tends to drain spontaneously, and a recurrent periareolar fistula is a complication in about 20 % of cases (see Sect. 8.3).
Other conditions, secondary to persistent nipple discharge or stagnant secretions, may be occasionally observed as persistent crusting of the nipple, dermatitis or eczema of the areola (see Sect. 11.1).
Finally, there are a number of other chronic inflammatory conditions, such as lymphocytic mastopathy and granulomatous mastitis, which may be unrelated, but which also may overlap with periductal mastitis. If that were true, at least some cases are best managed by surgery directed to proximal ectatic ducts.
PATHOGENESIS. The pathogenesis of duct ectasia/periductal mastitis complex is doubtful, given that it can be considered from three different points of view.
A primary dilatation of the ducts due to hormone-induced muscle relaxation or to hypersecretion or failure of absorption of duct fluid. According to this classic theory, duct ectasia is the primary event, leading to stagnation of secretion, epithelial ulceration and leakage of duct secretions containing chemically irritant fatty acids into periductal tissue to give a chemical inflammatory process. This sequence starts when one or more of the larger ducts dilate, reaching a diameter of 5 mm in gross examples. This is commonly restricted to the portion of the duct deep to the areola. Typically, three or four ducts are dilated, the remaining ducts being normal. In a few cases, dilatation extends peripherally to involve segmental and even subsegmental ducts.
An obstructive phenomenon due to squamous metaplasia, either congenital or acquired with secondary duct dilatation and leakage of contents to give secondary inflammation. However, this view does not keep into account all aspects of the clinical pictures. Moreover the age distribution is contradictory if we consider the fact that inflammatory patterns classically occur in an earlier age group.
A primary inflammatory condition, autoimmune or due to bacterial invasion, with secondary duct wall destruction leading to dilatation. This alternative theory explains the primary process as periductal mastitis – perhaps on an autoimmune basis – leading to weakening of the muscle layer of the ducts and secondary dilatation.
The pathogenesis of combined duct ectasia and periductal mastitis is a more difficult problem, and it is likely that more processes may occur separately or in conjunction, thus explaining the wide spectrum of clinical presentations. The basic classic sequence – not valid in all cases – is set out below.
1.
Duct ectasia with ducts filled with stagnant secretions. These may vary in colour and consistency and may be squeezed out leading to nipple discharge, usually of small amounts but sometimes sufficient to cause embarrassment.
2.
Ulceration of the epithelial lining of the ducts and ulceration, due to stagnation of secretions, that may result in blood-stained nipple discharge and in leakage of stagnant secretions into the periductal tissue.
3.
Inflammatory response, in the early stages chemical rather than bacterial, to secretions that contain fatty acids which are chemically irritant. Usually seen beneath the edge of the areola, dilatation may – but rarely does – extend into the subsegmental ducts, more peripherally in the breast, where stasis could contribute to the growing of some chronic inflammatory conditions.
4.
Abscess formation – when this occurs, simple drainage is unlikely to be curative, and a persistent or recurrent fistula is likely to result. In some cases a massive fibrotic reaction stops abscess formation leading to a mass which may simulate a cancer.
5.
Fibrosis – the periductal inflammation leads to fibrosis, and, as the fibrous tissue matures and contracts, it leads to nipple retraction.
However, a number of clinical aspects are incompatible with the classic view of the disease, and it is unlikely that this sequence is correct. It seems more likely for the duct ectasia/periductal mastitis complex to encompass several different processes which nevertheless may coexist and interact in some cases.
In particular, there are distinct processes affecting the young and the old women and perhaps a third process affecting all ages. Nipple discharge in the premenopausal woman and nipple retraction of the postmenopausal woman may be separate variants, while younger women tend to demonstrate the full picture with the exception of nipple discharge. Perhaps there is a greater obstructive element in young women, and this minimises nipple discharge while improving leakage of duct contents into the periductal tissues.
Moreover, in order to provide a pragmatic approach to the understanding and management of the disease, a number of facts must be taken into consideration.
Simple duct dilatation with secretory stagnation can arise as an asymptomatic development, best regarded as an aberration of involution.
Pregnancy and breastfeeding have frequently been considered as important in the aetiology of duct ectasia; however, this relation is hardly noticeable.
Periductal fibrosis can occur in the absence of duct ectasia or of inflammation and probably represents part of the normal involutional process in the breast.
The dilatation, too, may result from the same periductal inflammation that may cause duct fibrosis or obliteration, from the effect of some hormones or from simple muscle atrophy.
The inflammatory complications are more common in young women, while nipple discharge and nipple retraction often occur in older women without overt inflammatory episodes.
Acute inflammation occurs frequently in young women with a congenital inverted nipple, but in other subjects of this same age group, inflammation leads to retraction of a previously normal nipple.
Some patients with recurrent subareolar abscess show a definite squamous metaplasia of the terminal duct, while a similar clinical picture can develop in women with grossly ectatic ducts without evidence of squamous epithelial replacement of the terminal ducts.
The mammary duct fistula associated with squamous epithelial replacement is one entity, probably congenital in most cases and then usually associated with inverted nipple, while in other cases the squamous epithelium may occur as a secondary down-growth.
There is increasing evidence for a bacterial role in severe forms of periductal mastitis, although at present this would appear to be secondary rather than primary.
In conclusion, firstly, clinical manifestations of minor degree can be regarded as a benign breast disorder, while they become a disease only when complicated by the development of severe inflammation. Then, whether it is primarily the inflammation leading to duct damage and dilatation or the duct dilatation giving rise to ulceration and leakage of contents still cannot be determined. There is evidence that both mechanisms are involved, by one or other in some cases, and together in others. Finally, it is also difficult to be certain at what stage a bacterial infection becomes more important than a chemical reaction to leaking duct contents. In each case, a symptomatic disease – inflammation, nipple discharge or retraction – must be regarded as the tip of the iceberg of a subclinical histological change.
CLINICAL SPECTRUM. In the mammary duct ectasia/periductal mastitis complex, all common breast symptoms are detectable either alone or interconnected (Fig. 9.3). Pain, usually noncyclical, is discussed in Chap. 7. Nipple Discharge in plain duct ectasia can be coloured, thick, creamy, or temporarily bloody (see Sect. 10.1). If inflammation is present, the most common complaint is a small amount of purulent discharge, which is confirmed by the patient expressing material from the nipple.
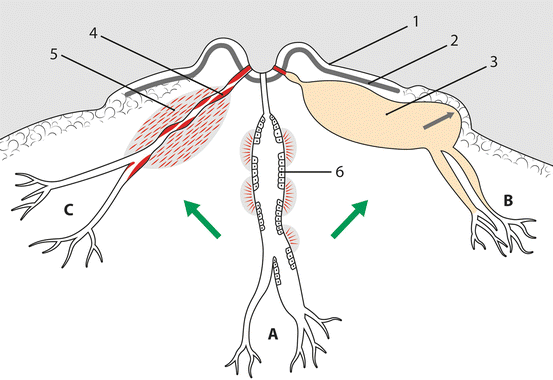

Fig. 9.3
The classic, albeit questionable, view of the pathogenesis of the clinical spectrum of mammary duct ectasia/periductal mastitis complex. (a) The stagnant secretions lead to patchy mucosal ulceration, and the contents leak through giving a chemical inflammatory response into periductal tissue. (b) Blockage of duct, sometimes but not always produced by squamous debris, leads to dilatation of the subareolar portion; because of the tough muscle of the areola, an abscess tends to burst through the skin at the edge of the areola (black arrow). (c) Inflammation leads to fibrosis of the duct wall that contracts producing more nipple retraction and (rarely) a chronic mass. 1, inverted nipple; 2, muscle of the areola; 3, subareolar abscess; 4, duct thickened by fibrosis; 5, fibrotic chronic mass (clinically rare); 6, schematic representation of the epithelial lining of the duct, with patchy ulcerations
Evanescent mass. It is a common presentation of periductal mastitis. The patient notices a small, slightly tender mass in the subareolar region. By the time she is seen in a clinic 7–10 days later, the mass has often disappeared. Such rapid development and regression of a breast mass is uncommon in any other breast condition. These masses, tender and not fixed to surrounding tissues, are typically 1–2 cm in diameter and may progress to reddening of the overlying skin and still regress in a few days. The patients have often been given antibiotics and naturally attribute their improvement to the treatment.
An evanescent mass may not recur or have a tendency to do so at the same site at intervals of a few months to 10 years or more. The recurrent mass has a tendency to become more severe with each recurrence. There is an appreciable incidence of bilateral involvement, and it is not uncommon for the opposite breast to become involved shortly or years after successful recovery of one breast.
A persistent mass. It endures for some weeks, and it is usually firm and fairly well defined. Cancer cannot be excluded, but cytological appearance (foamy macrophages and inflammatory cells without epithelial cells) is highly characteristic and justifies a short course of appropriate antibiotics. If the mass does not resolve rapidly, core needle or excisional biopsy is desirable in women of cancer age group. Provided there is no overt abscess formation, a periareolar biopsy wound will heal satisfactorily, and there is no need to perform a formal duct excision. In fact, macroscopically dilated ducts are not particularly common in the presence of a simple periductal mastitis mass.
Subareolar abscess. Any of the above subareolar masses may proceed to abscess formation (see Sect. 8.3). The underlying mass becomes attached to the skin, which first becomes reddened and then shows bluish discolouration. Nipple retraction will often develop if not already present, and nipple oedema may be marked. These abscesses are associated with discomfort which varies from mild to severe, but not usually as severe as with pyogenic abscess. Aspiration will yield creamy or dirty, watery pus, and bacteriological culture may be sterile on the first occasion. If not treated, it will burst spontaneously with considerable relief, but a persistent sinus will remain, or the abscess will recur sooner or later and usually at the same site.
Mammary Duct Fistula (see Sect. 8.3). It may result after simple drainage with persisting discharge or recurrent abscesses presenting at the same point.
Fibrosis. It is usually the ultimate result of inflammatory process. As fibrous tissue matures and contracts, it leads at minimum to nipple inversion/retraction. Therefore, even if a high incidence of congenital nipple inversion in young girls with recurrent subareolar abscess is common, a more progressive retraction of a previously normal (or already inverted) nipple during the evolution of severe periductal mastitis is commonly observed.
In few cases, fibrosis yields a hard oedematous chronic mass, fixed to the skin and with nipple retraction, sometimes with axillary node enlargement. The lesion simulates cancer closely, so that it may be impossible to distinguish the two on mammography, but core needle biopsy will yield the typical macrophages and inflammatory cells with no epithelial cells, so a presumptive diagnosis can be made and a trial of antibiotics given before biopsy. In these cases, the typical large ducts with their pultaceous contents are more likely to be present. A formal duct excision procedure under appropriate antibiotic cover, together with excision of the mass, is usually indicated.
9.2.3 Other Nonproliferative Lesions
Other nonproliferative lesions do not usually cause a palpable mass but can cause changes that are seen on cytology of discharge (as papillary apocrine changes) or on mammogram (as calcifications or minimal opacities corresponding to areas of mild hyperplasia).
Papillary apocrine change is a proliferation of ductal epithelial cells showing apocrine features, characterised by eosinophilic cytoplasm.
Epithelial–related calcifications are benign calcifications that are observed in the breast tissue and can be seen in normal ducts and lobules, stroma or blood vessel walls.
Mild hyperplasia of the usual type is an increase in the number of epithelial cells within a duct that is more than two, but not more than four, cells in depth. With five or more cells in depth, the hyperplasia of the usual type is defined as ‘moderate’. Mild hyperplasia is associated with no risk of developing cancer, whereas the moderate form shows a slightly increased risk (1.5–2 times). The pattern of epithelial cells is very close to normal.
9.3 Proliferative Lesions Without Atypia
Clinical Practice Points
The growth patterns of fibroadenoma and related subtypes may have static or regression phases.
Biologic behaviour is very constant in hamartomas, while it may change from benign to malignant in phyllodes tumours.
Papillary lesions of the breast incorporate a large spectrum of benign (solitary papilloma, multiple papillomas, papillomatosis, juvenile papillomatosis) as well as malignant lesions, relatively uncommon and still not well understood.
In solitary papilloma atypical hyperplasia is less commonly seen, if compared to multiple papillomas.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








