Fig. 7.1
B cell development, subset, and differentiation. In the bone marrow, the B cell lineage cell starts as the pro-B cell, which differentiates into the pre-B cell expressing the pre-BCR on the cell surface. Immature B cells that escape this process start expressing IgD and emerge in the periphery as transitional B cells (T1/T2). Mature B cells are classified into B-1 cells and B-2 cells. B-2 cells include follicular (FO) B cells and marginal zone (MZ) B cells. FO B cells are activated through the interaction with antigen-presenting cells and T cells. Some B cells directly differentiate into short-lived plasma cells by clonal expansion and produce IgM promptly. Other B cells migrate into the primary follicle, differentiate into centroblasts, and initiate the germinal center reaction. Somatic hypermutation occurs in centroblasts, and those that undergo somatic mutation resulting in high-affinity antibody production are positively selected, expand, and differentiate into centrocytes. Centrocytes undergo isotype switching, acquire different Ig isotypes, and differentiate into memory B cells or plasmablasts. Some become long-lived plasma cells in a niche in the bone marrow. MZ B cells also undergo clonal expansion and become plasma cells. B-1 cells have a distinct ontogeny in mice
The other B-2 cell subset is the marginal zone B cell [11]. In mice, MZ B cells express the phenotype of IgMhiIgDlowCD21hiCD23−CD24dim. They are located in the marginal sinus region (marginal zone) of the spleen. MZ B cells also originate from T2 B cells, although how their fate is determined (FO vs. MZ) is not fully elucidated [12]. MZ B cells are vital in the general first-line defense against blood-borne antigens. Upon recognition of T cell independent antigens they start to proliferate and differentiate into plasma cells that produce low-affinity antibodies. Thus, MZ B cells function as “innate-like lymphocytes” [13]. Human MZ B cells express IgMhiIgDlowCD1c+CD21hiCD23–CD27+ phenotype [13, 14]. The ontogeny and functions of human marginal zone B cells remain controversial. B cells identical to splenic MZ B cells are also present in human peripheral blood, suggesting that human MZ B cells recirculate [15], unlike murine marginal zone B cells that are restricted to the spleen [13]. Also, a significant proportion of IgM+CD27+ MZ B cells have been shown to undergo somatic mutations in humans [16].
B-1 B cell is the third type of mature B cells with a distinct cell-surface phenotype, at least in mice [17, 18]. B-1 B cells are predominantly present in the pleural and peritoneal cavities. Additionally, there is a very small population of B-1 cells in the spleen. B-1 B cells are further divided into two subpopulations, B-1a (CD11b+CD5+) and B-1b cells (CD11b+CD5−). B-1a cells develop mainly from fetal-liver–derived haematopoietic stem cells. B-1 B cells have a unique self-renewing capacity. B-1 B cells preferentially produce polyreactive IgM and IgA, and like MZ B cells known as “natural antibodies,” play an important role as “innate-like B cells.” Whereas the B-1 B cell population is distinct in mice, the existence of human B-1 B cells has been controversial. However, recent studies have demonstrated CD20+CD27+CD43+CD70− as a human B-1 cell phenotype [19].
7.4 B Cell Activation
B cells respond to diverse chemical and environmental cues. BCR can bind a vast variety of antigens, including native proteins, glycoproteins, and polysaccharides. Mature B cells are essentially activated by the recognition of antigen. Classically, B cell activation is classified into T-dependent activation and T-independent activation. In T-dependent response, the B cell is activated by antigen and also by T-cell interaction through CD40/CD40L [20]. T-independent antigens can induce B cell activation without T-cell help.
B cell fate is determined by BCR signaling [21]. Transmembrane Ig serves as the antigen-binding subunit of the BCR. Ig expressed on the cell surface is noncovalently associated with the CD79a (Igα) and CD79b (Igβ) heterodimer, which serves as a signal transducing subunit [22, 23]. CD79a and CD79b cytoplasmic domains contain immunoreceptor tyrosine-based activation motifs (ITAMs). When a B cell encounters antigen, ITAMs within CD79a/CD79b are phosphorylated by Src-family protein tyrosine kinases (PTKs), Lyn, Fyn, and Blk [24]. Whereas Lyn is a predominant Src-family PTK in B cells [25, 26], Blk is also a B cell-specific Src-family PTK. Blk deficiency in mice does not exhibit an overt abnormal phenotype, however, Blk polymorphisms are closely associated with several connective tissue diseases including SLE [27]. Phosphorylated ITAMs then recruit other PTKs, such as Syk and Btk, which further phosphorylate the ITAMs, as well as other signaling molecules, that mediate downstream signaling events which determine the fate of the cell during development, activation, and proliferation. Among them, BANK1 is an adaptor protein primarily expressed in B cells. BANK1 polymorphism is also associated with SLE and systemic sclerosis [28, 29].
B cell responses are further fine-tuned by other surface molecules (Fig. 7.2). These molecules are roughly categorized into positive regulators and negative regulators. Positive regulators include CD19, CD40, and CD45. CD19 is a cell-surface protein expressed in B cells and follicular dendritic cells. CD19 is associated on the B-cell membrane with CD21. CD21 is the receptor for C3d and Epstein–Barr virus [30]. CD21 generates transmembrane signals through CD19 and informs the B cell of inflammatory responses within microenvironments. Once the complement is activated, C3d-bearing antigens efficiently activate B cells as BCR and CD19 are colligated [31]. CD19 has nine conserved tyrosine residues in the cytoplasmic domain. Lyn phosphorylates these tyrosines, which then mediate PI3-kinase activation and Lyn kinase activity amplification [32, 33].
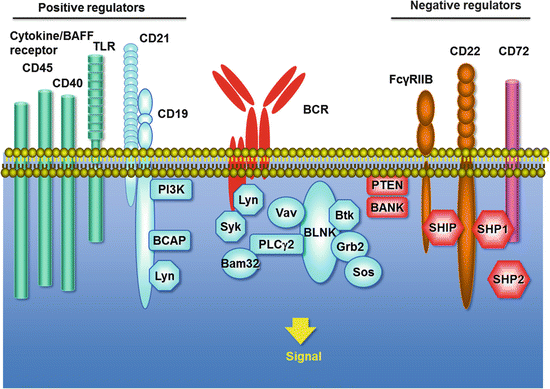

Fig. 7.2
B cell signaling. The BCR consists of membrane-bound Ig associated with the CD79a (Igα) and CD79b (Igβ) heterodimer. Upon BCR ligation, Src-family protein tyrosine kinases (Lyn etc.), Syk, and Btk are activated, and mediate downstream signaling cascades that determine the fate of the cell during the development, activation, and proliferation. These responses are further fine-tuned by B-cell surface molecules, which are roughly categorized into positive regulators and negative regulators. Positive regulators include CD19/CD21, CD40, CD45, Toll-like receptors (TLR), and cytokine receptors including BAFF-R. Negative regulators include CD22, CD72, and Fcγ receptor IIB (FcγRIIB), which are characterized by ITIMs (immunoreceptor tyrosine-based inhibitory motifs) in the cytoplasmic domain
CD40 is expressed on B cells as well as a variety of cells including monocytes and dendritic cells. CD40 serves as a critical survival factor for GC B cells and is the ligand for CD154 (CD40 ligand; CD40L) expressed by activated T cells [20]. CD40 ligation by CD40L promotes B-cell activation, maturation, differentiation, and Ig production. It is critical to germinal center formation and promotes memory B cell development. CD40/CD40L interactions rescue immature B cells from negative selection and germinal center B cells from apoptosis.
Toll-like receptors (TLRs) are also expressed on B cells [34]. Some TLRs, such as TLR2 and TLR4, which are triggered by lipothecoic acid and lipopolysaccharide (LPS), respectively, are present on the plasma membrane, whereas others, such as TLR7 and TLR9, which recognize single-stranded RNA and CpG-containing DNA, respectively, are present in the endosomal compartment. TLR, alone or with other stimuli, can activate B cells. Although human naïve B cells do not express significant levels of TLRs, human memory B cells constitutively express TLR2, TLR6, TLR7, TLR9, and TLR10 [34–36].
B cell response is also regulated by cytokines, such as IL-4, IL-5, IFN-γ, and TGF-β. These cytokines differentially induce a class switch. IL-21 secreted by follicular dendritic T cells is also important for germinal center formation in addition to IL-4 [37].
B-cell activating factor (BAFF) , a member of the tumor necrosis factor family of cytokines, is particularly important in B-cell survival and differentiation [38]. BAFF is produced by DCs and monocyte/macrophages and binds three receptors, B cell maturation antigen (BCMA), transmembrane activator and calcium-modulator and cyclophilin ligand interactor (TACI), and BAFF-R. The BAFF signal induces immature B-cell survival and mature B-cell proliferation within peripheral lymphoid tissues when coupled with BCR ligation. Transgenic mice that overexpress BAFF develop a lupus-like disease. Belimumab, a monoclonal antibody against BAFF, has been approved for the treatment of SLE by the Food and Drug Administration (FDA).
By contrast, there are several negative regulators that dampen B cell activation signals [39]. CD22 functions as a mammalian lectin for α2,6-linked sialic acid [40]. CD22 has ITIMs (immunoreceptor tyrosine-based inhibitory motifs) in the cytoplasmic domain that recruit SHP-1 phosphatase. CD19 and CD22 reciprocally regulate their functions, and their balance appears to determine the susceptibility of autoimmunity [41]. CD72 also functions as a negative regulator of signal transduction through the ITIM motif and as the B-cell ligand for Semaphorin 4D (CD100) [42]. Fcγ receptor IIB (FcγRIIB) , which binds the Fc portion of IgG, is another important negative regulator of B cells that contains an ITIM [43].
Other important B cell-surface proteins include CD20, a mature B cell-specific molecule that functions as a membrane-embedded Ca2+ channel, although their specific significance remains unclear [44]. Note that CD20 is the target of ritixumab, a chimeric CD20 mAb which was approved first by the FDA for clinical use in cancer therapy. CD23 is a low-affinity receptor for IgE expressed on activated B cells. CD24 is a pan-B-cell molecule, although this unique GPI-anchored glycoprotein’s function remains unknown. CD38 is a membrane-associated enzyme that may increase BCR signaling by its interaction with CD19.
7.5 Antibodies
The B cell is a central player of humoral immunity by its capacity to differentiate into cells that can secrete antibodies. Antibodies are the secreted form of the BCR. Antibodies, i.e., Igs, are tetrameric molecules composed of two pairs of polypeptide chains (Fig. 7.3). Ig has two identical light chains with a molecular weight of 25 kD and two identical heavy chains with a molecular weight of 50–75 kD. There are two types of light chains, kappa (κ) and lambda (λ). Kappa chains are more common than λ chains in humans (60 %) and mice (95 %), although no functional differences have been found between the κ and λ chains. Antibody molecules consist of three portions presenting a roughly Y shape. Two arms of the Y are identical and termed Fab fragments containing a variable region of the light and heavy chains. The light and heavy chains of Fab fragments consist of VL and CL domains and VH and CH1 domains, respectively. Antigen-binding sites are formed by the paired VL and VH domains. The variable regions are responsible for the recognition of a great diversity of antigens. The tail of the Y is termed the Fc fragment and consists of constant regions, CH2 and CH3 domains. IgM and IgE have an extra C domain. The constant regions of the heavy chains determine the Ig class or isotype. The antibody molecule exerts effector functions such as complement activation and binding to Fc receptors. These functions vary significantly with antibody class. There are five known isotypes in mice and humans: IgM, IgG, IgA, IgE, and IgD (Table 7.1).
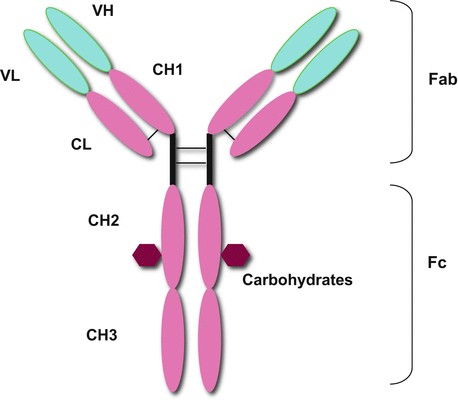

Fig. 7.3
The structure of antibody. Antibodies (Igs) are tetrameric molecules composed of two pairs of polypeptide chains, presenting roughly a Y shape. Ig has two identical light chains and two identical heavy chains. Antibody molecules consist of three portions. Two arms of the Y are termed Fab fragments containing a variable region of the light and heavy chains. The light and heavy chains of Fab fragments consist of VL and CL domains and VH and CH1 domains, respectively. The variable regions are responsible for the recognition of great diversity of antigens. The tail of the Y is termed Fc fragment consisting of constant regions, CH2 and CH3 domains
Table 7.1
Structure of immunogloburin G
Ig isotypes in humans | ||||||||
|---|---|---|---|---|---|---|---|---|
IgM | IgG1 | IgG2 | IgG3 | IgG4 | IgA | IgE | IgD | |
Molecular weight (kD) | 970 | 146 | 146 | 165 | 146 | 160 | 188 | 184 |
Half-life (day) | 10 | 21 | 20 | 7 | 21 | 6 | 2 | 3 |
Neutralization | + | ++ | ++ | ++ | ++ | ++ | − | − |
Opsonization | + | ++ | +/− | ++ | + | + | − | − |
Sensitization for killing by NK cells | − | ++ | − | ++ | − | − | − | − |
Sensitization of mast cells | − | + | − | + | − | − | ++ | − |
Activation of complement system | +++ | ++ | + | ++ | − | + | − | − |
Transport across epithelium | + | − | − | − | − | ++ | − | − |
Transport across placenta | − | ++ | + | ++ | +/− | − | − | − |
Diffusion into extravascular sites | +/− | ++ | ++ | ++ | ++ | + | + | − |
Mean serum levels (mg/ml) | 1.5 | 9 | 3 | 1 | 0.5 | 3 | 0.0003 | 0.03 |
IgM accounts for approximately 10 % of the serum Ig pool. It is the first Ig generated during the primary immune response and thus plays a pivotal role in the primary immune response. IgM forms a pentamer in serum, which helps to enhance its avidity for antigen as IgM antibody generally has low affinity.
IgG is the most abundant Ig isotype that makes up approximately 75 % of the serum Ig pool. In humans, there are four subclasses of IgG: IgG1, IgG2, IgG3, and IgG4. IgG1 is the most abundant (>50 % of total IgG) and the major component of the response to protein antigens, whereas IgG2 is produced in response to polysaccharide antigens. IgG3 is considered to be important in the response to respiratory viruses. IgG4 is the least abundant (<5 %) and undergoes “Fab-arm exchange,” resulting in bispecific antibody. IgG4 is associated with Th2 response, as is IgE. Functional properties are different among the subtypes based on the Fc difference. IgG1 and IgG3 strongly activate the complement system and can bind to mononuclear cells and neutrophils. The half-life of IgG3 (7 days) is shorter than that of the other subtypes (21 days). IgG is the major Ig that causes autoimmune diseases as it can penetrate into tissues. Recently, accumulated evidence has established a concept of “IgG4-related disease” characterized by a lymphoplasmacytic infiltrate composed of IgG4+ plasma cells [45], although the molecular mechanisms of how IgG4 participates in the pathogenesis remains unclear.
IgA is the predominant isotype at mucosal surfaces [46]. It makes up approximately 15% of the serum Ig pool. IgA exists as two subclasses, IgA1 and IgA2. IgA1 is mainly a monomer in serum, and IgA2 exists as a dimer linked by a J chain and linked to a peptide known as a secretory component. IgA binds to pIgR and transits through secretory epithelium. Having this component, polymeric IgA is resistant to enzymatic degradation. IgA activates complement through the alternative pathway. IgA deposition is observed in Henoch–Schonlein purpura. IgA antibodies also have a pathogenic role in mucous membrane pemphigoid and herptiform dermatitis.
IgE is the least abundant Ig in the serum of nonatopic individuals [47]. IgE is mainly sequestered in tissues and binds in the monomeric form to the high affinity FcεR on basophils and mast cells. Antigen binding to cell-bound IgE leads to degranulation and release of mediators which causes immediate hypersensitivity reactions, such as urticarial and anaphylaxis. Increased IgE levels are required for the upregulation of the expression of the high-affinity IgE receptor (FcεRI) by mast cells homing to mucosal surfaces.
IgD is also less abundant in serum. IgD is expressed at high density on the mature B cell surface, and induces strong BCR signals. Its physiologic function as a soluble form is not well understood, however, a recent study has demonstrated that IgD binds to basophils and mast cells and activates these cells to produce antimicrobial factors to participate in a respiratory immune defense in humans. In an autoinflammatory syndrome, “hyper-IgG syndrome,” patients present increased levels of serum IgD, although the mechanism remains unknown.
Antibodies recognize entering foreign antigens and trigger a biological response to eliminate the antigen utilizing three types of response (Fig. 7.4). The first, known as neutralization, antibodies prevent the adherence of pathogens and toxins to host cells. Second, antibody coats the surface of a pathogen, and promotes phagocytosis, which is called opsonization. Antibodies bound to the pathogen are recognized by Fc receptors expressed on phagocytes [43]. Third, antibodies coating a pathogen can activate the complement system by classical pathways. Complement proteins bound to the pathogen surface enhance opsonization and lyses some bacteria [48].
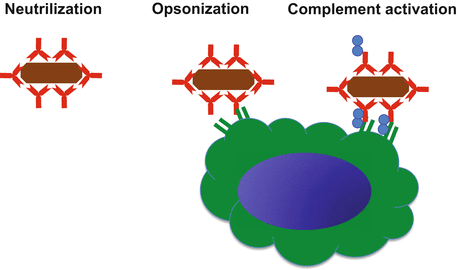

Fig. 7.4
Functions of antibody. Antibodies recognize antigens (brown) and trigger a biological response in three ways. (i) Neutralization, antibodies prevent the adherence of pathogens and toxins to host cells. (ii) Opsonization. Antibody coats the surface of a pathogen, and promotes phagocytosis. Antibodies bound to the pathogen are recognized by Fc receptors expressed on phagocytes (green cell). (iii) Complement activation. Complement proteins (blue) bound to the pathogen surface with antibody. Antibody enhances opsonization and lyses some bacteria
7.6 Cytokines
In addition to antibody production, antibody-independent functions of B cells have been identified. These include antigen presentation, costimulation, and lymphoid tissue neogenesis and structure regulation. B cells can produce a variety of cytokines [49]. They secrete cytokines constitutively, or in response to antigen, TLR ligands, T cells, or their combinations [50]. Cytokines produced by B cells include proinflammatory cytokines such as IL-1, IL-2, IL-4, IL-6, IL-12, IL-13, IFN-γ, TNFα, and LTα, hematopoietic growth factors such as GCSF, GMCSF, and IL-7, and immunosuppressive cytokines such as TGFβ and IL-10 [51]. It has been proposed that cytokine-producing B cells can be subdivided into B effector-1 (Be-1) cells and B effector-2 (Be-2) cells based on their cytokine profile in analogy with Th1 and Th2 cells [52]. B cells primed by TH1 cells and antigen differentiate into Be-1 cells capable of producing cytokines associated with TH1 responses, such as IFN-γ and IL-12. Be-1 cells do not secrete substantial amounts of IL-4, IL-13, or IL-2, but can secrete IL-10, TNF, and IL-6. Conversely, B cells primed by TH2 cells and antigen differentiate into Be-2 cells that produce cytokines associated with TH2 responses, such as IL-2, IL-4, and IL-13. Be-2 cells produce minimal amounts of IFNγ and IL-12, but can also secrete IL-10, TNF, and IL-6 [50]. Current data suggest that effector B cells are derived from conventional FO B cells [53, 54]. Finally, B cells that only produce immunosuppressive IL-10 are called regulatory B cells, or B10 cells, and are discussed in the next section.
Effector B cells can also modulate T cell mediated immune responses. In mice, Be-1 and Be-2 B cells generated in vitro can promote the in vitro activation and differentiation of naive T cells into effector TH1 and TH2 cells, respectively, through a cytokine-dependent mechanism [52]. Similarly, IL-12-producing human B cells and IL-4-producing human B cells are shown to promote TH1 and TH2 cell responses, respectively [55–57].
7.7 Regulatory B Cell
In addition to the effector B cell subsets that enhance immune responses, there are also certain B cell subsets exhibiting regulatory functions [58, 59]. The first evidence for the suppressive function of B cells was documented in the 1970s. Depletion of B cells from splenocyte preparations eliminated the ability of adoptively transferred cell preparations to inhibit DTH responses in guinea pigs [60]. Adoptive transfer of Ag-activated B cells or B-cell blasts could also induce tolerance in recipient naive mice and induce the differentiation of suppressor T cells [61–63]. In 1996, a protective function of B cells was demonstrated against experimental autoimmune encephalomyelitis (EAE), a mouse model for human multiple sclerosis, in B10.PL mice [64]. The B cell-deficient μMT mice were unable to recover from EAE. Another study elucidated that the exacerbation of EAE in μMT mice is due to a deficiency in IL-10-producing B cells [65]. The term “regulatory B cells” was first used in 2003 to designate B cells with inhibitory properties [66, 67]. Regulatory B cells mediate their diverse regulatory functions mainly via the production of IL-10. In mice, splenic CD1dhiCD5+ B cells are shown to exert potent immunosuppressive function through IL-10 production, and are thus termed “B10 cells” [68]. These “B10 cells” have a phenotype resembling MZ B cells. T2-MZ precursor cells (CD19+CD21highCD23+CD24highCD93+) have also been proposed as regulatory B cells [59]. Therefore, it is likely that regulatory B cells are derived from the MZ B cell lineage within B2 cells in mice. Additionally B1 cells may also exert immunosuppressive effects via IL-10. In humans, CD19+CD24hiCD27+ B cells and CD19+CD24highCD38high B cells are proposed as regulatory B cells [69, 70].
Regulatory B cells have been shown to play a potent suppressive function in various murine disease models. In skin inflammatory diseases, regulatory B cells have been reported to suppress inflammatory reactions in contact hypersensitivity in mice [71]. B cell infiltration is observed at the site of CHS in mice [72]. Regulatory B cells also appear to have a critical role in murine connective tissue disease models including arthritis, lupus, and scleroderma [73–75].
7.8 B Cell and Disease
B cells play a critical role in host defense against various infections. This is mainly mediated by antibody production, but nonantibody functions may be also important. Genetic defects in molecules expressed in B cells and/or other cells cause inherited immunodeficiency related to B cell dysfunction [76]. Btk deficiency results in Bruton’s X-linked agammaglobulinemia, and CD40/CD40L deficiency results in hyper-IgM syndrome, which are among the best examples. Immunodeficiency caused by genetic defects in BLNK, CD19, ICOS, and AID is also reported [76].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








