Fig. 12.1
Tense blisters and extensive postinflammatory pigmentary changes in a child with long-standing chronic bullous disease of childhood
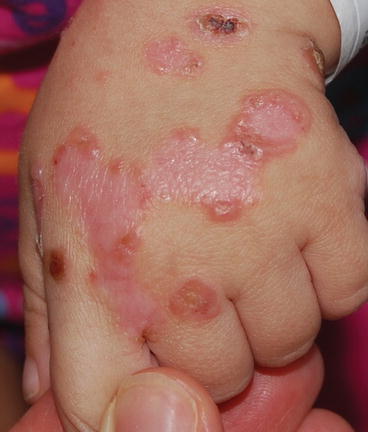
Fig. 12.2
Tense blisters in a “string of pearls” configuration on the dorsal hand of a child with chronic bullous disease of childhood
Most cases of CBDC in children are idiopathic; but infections (Salmonella enteritis [5], Epstein- Barr virus [6], Group A streptococcal pharyngitis, hepatitis A [7]) and medications (amoxicillin-clavulanic acid [8], trimethoprim-sulfamethoxazole [9], vancomycin [10]) have been reported as possible triggers. It is important to inquire about potential triggers, especially medications, since discontinuation of the medication leads to resolution.
Diagnosis
The diagnosis of CBDC may be suspected clinically in typical cases (young child, lower abdomen and perineal involvement, bullae in “string of pearls” configuration), but direct immunofluorescence (DIF) testing is necessary for confirmation. Histological examination is usually not sufficient for diagnosis in that it can resemble dermatitis herpetiformis or bullous pemphigoid. Hematoxylin-eosin stained biopsy from involved skin demonstrates a subepidermal blister along with a predominantly neutrophilic infiltrate, with or without eosinophils, in the papillary dermis. Papillary neutrophilic microabscesses may be detected, especially if serial sections are studied [11]. Direct immunofluorescence of perilesional skin displays linear deposits of IgA at the dermal-epidermal junction, which is diagnostic for CBDC [12]. Weaker bands of IgG, IgM, and C3 are also occasionally present (some refer to this as mixed bullous disease of childhood) [13]. In equivocal cases, indirect immunofluorescence (IIF) will identify circulating IgA antibodies, usually low titers, in approximately 70 % of children [1, 14].
Treatments
Dapsone and Sulfapyridine
Dapsone or sulfapyridine is the treatment of choice for CBDC. Dapsone is a sulfone antibiotic approved for the treatment of dermatitis herpetiformis and leprosy in pediatric patients (lower age limit not specified). Sulfapyridine is a sulfonamide antibiotic that is no longer available in the United States, but is available in other countries. Although there are no controlled or comparative studies, there is ample anecdotal evidence in the form of case reports and case series to support the use of dapsone or sulfapyridine as monotherapy for the treatment of CBDC [7, 11, 13, 15, 16]. The response to treatment is usually rapid, with resolution of blisters within 2 weeks of starting therapy. Kenani et al. [10] performed a retrospective review of 25 children with CBDC. Nineteen children were treated with dapsone alone (1–2 mg/kg) and 11 of 19 patients achieved disease control after 8–15 days of therapy. Eight patients required the addition of systemic corticosteroids (0.5–1 mg/kg) to control their disease. The duration of treatment ranged from 3 to 60 months. Seventeen patients had achieved remission at the time of publication. Four patients developed methemoglobinemia that necessitated a reduction in dose or withdrawal of medication for a few days, but no other adverse effects were seen. Wojnarowska et al. [1] studied a series of 25 children with CBDC. All children were treated with either dapsone (20–200 mg) or sulfapyridine (0.5–2 g) and all had a response to treatment within 72 hours with relapses when the drug was withdrawn. Sixteen of 25 patients achieved remission over a variable time period of 6 months to 16 years (mean 5.3 years). Eight children were also treated with low dose systemic corticosteroids. Detailed treatment information and side effects were not provided. Marsden et al. [15] reported 20 children with CBDC treated with either dapsone (20–200 mg) or sulfapyridine (0.25–3 g); 12 of 20 children were well controlled with monotherapy and eight children required the addition of corticosteroids.
Corticosteroids
Again the evidence is anecdotal, but it is apparent from the literature that systemic corticosteroids are useful as adjunctive therapy for disease that is resistant to first line treatment and severe disease presentations (e.g., extensive blistering and erosions, severe pruritus, symptomatic mucous membrane involvement), but should be avoided as monotherapy due to the long-term side effects in children [3, 7, 10, 17, 18]. The most frequently used dose is 0.5–1 mg/kg (prednisone/prednisolone). The corticosteroids are continued until the disease is controlled, then they are tapered off, usually over a 3–6 week period. They can then be used intermittently for severe exacerbations if necessary. Wojnarowska et al. [1] used low dose systemic steroids (<10 mg; weight-based dosing not provided) in 8 of 25 children not controlled on dapsone or sulfapyridine and concluded that the value of the addition of low dose systemic corticosteroids is difficult to determine as the effect was neither rapid nor dramatic. If systemic corticosteroids are to be used, it appears at least moderate doses (0.5–1 mg/kg) are necessary.
Medium-potency topical corticosteroids have been reported to be useful as monotherapy for controlling very limited and mild disease [7]. Topical corticosteroids are frequently used as adjunctive therapy with systemic agents for symptom control.
Antibiotics
The semisynthetic penicillins (oxacillin, cloxacillin, dicloxacillin, and flucloxacillin), macrolide antibiotics (erythromycin) and sulfonamides (trimethoprim-sulfamethoxazole) have all been reported to be useful for the treatment of CBDC [3, 10, 19–26]. Alajlan et al. [19] conducted a prospective observational study of seven children with confirmed CBDC treated with flucloxacillin (similar to dicloxacillin). Four of seven patients had a complete response to flucloxacillin and the medication was stopped after 2.5–4 months of therapy with no relapses. The remaining three patients had a complete response, but relapsed when flucloxacillin was stopped and have remained on treatment for 3–6 years. Interestingly, the patients treated within 1 month of disease onset responded better to flucloxacillin then patients with longstanding disease. Periodic monitoring of complete blood count (CBC) and liver function was done and no laboratory abnormalities or adverse effects were seen. An additional nine patients treated with semisynthetic penicillins have been reported with similar results [10, 20–22]. Erythromycin was first reported as an effective treatment by Cooper et al. [23]. A 5-year-old girl with CBDC with + IgA and IgG on DIF and IIF was treated with erythromycin with complete control of her disease within 2 weeks. Discontinuation of erythromycin led to a relapse of blisters and therefore she was on continuous therapy at least 24 months. Other authors have reported similar results [3, 10, 21]. A survey of members of the British Society for Pediatric Dermatology detailed 13 patients treated with erythromycin; used as monotherapy in five children and in combination with other agents (dapsone, prednisolone, nicotinamide, sulfamethoxypyridazine) in eight children. One patient had complete resolution, three had good improvement but relapsed, five patients had some improvement and four patients had minimal response. This data suggests that erythromycin alone is unlikely to produce a sustained remission [24]. Two patients cleared with trimethoprim-sulfamethoxazole within 1 week with no relapse and one cleared within 2 years [3, 25]. Pulimood et al. [26] reported a 2-year-old boy who continued to develop blisters despite dapsone 2.5 mg/kg for 3 weeks. The addition of trimethoprim-sulfamethoxazole led to resolution of blisters in 4 days and complete resolution of disease in 10 months. It is hypothesized that antibiotics are effective due to inherent anti-inflammatory and not antimicrobial properties. Antibiotics have a more favorable side effect profile than dapsone and systemic corticosteroids, require less frequent laboratory monitoring and may be a good alternative in select patients with mild to moderate disease.
Colchicine
Colchicine is an anti-inflammatory drug that is FDA approved in children 4 years and older for the treatment of familial Mediterranean fever. Colchicine appears to be a well-tolerated and effective treatment for CBDC and can be considered an alternative therapy in children with glucose-6-phosphate dehydrogenase deficiency (G6PD) or who develop intolerable side effects from dapsone [27–29]. Banodkar and Al-Suwaid [27] treated eight children with G6PD deficiency or steroid-dependent disease with colchicine 0.5 mg twice a day. Within 4–6 weeks of colchicine therapy, five patients showed a complete response without the need for corticosteroid therapy. Three patients had a good response, but still required corticosteroids, although at lower doses, to maintain remission. Two additional patients were reported with similar responses. One patient developed diarrhea, but overall colchicine was very well tolerated with no other adverse effects. No abnormalities on CBC or liver and renal function tests were seen.
Mycophenolate Mofetil
Mycophenolate mofetil is an immunosuppressive agent that preferentially inhibits T-lymphocytes and has been reported to be an effective steroid-sparing agent in adults with immunobullous disease [30]. It is approved in infants and children for the prevention of renal allograft rejection. There is a single case report describing its use in a child with severe CBDC resistant to dapsone and requiring repeated pulses of corticosteroids for disease control [31]. Mycophenolate mofetil 310 mg/m2, later decreased to 155 mg/m2 due to lethargy and decreased oral intake, was initiated and she showed rapid improvement within a few days and was almost clear after 6 weeks of therapy with mycophenolate mofetil and dapsone without the need for additional corticosteroids.
Miscellaneous Treatments
Nicotinamide (30 mg/kg) plus dapsone was helpful in controlling CBDC in a 4-year-old girl who had not responded to high-dose dapsone and systemic corticosteroids over 3 months. Within 2 weeks she had complete resolution of her blisters and was successfully tapered over 12 weeks [32].
Tacrolimus ointment 0.03 % was reported to be helpful in a 4-year-old girl with CBDC who experienced a flare of blisters while on dapsone. The addition of tacrolimus ointment 0.03 % resulted in resolution of blisters within 2 weeks and was used periodically to control flares [33].
Thalidomide was reported in an 8.5-year-old boy with severe CBDC who required systemic steroids for disease control [34]. The patient developed iatrogenic Cushing syndrome after 1 year on corticosteroids. The disease responded to dapsone, but dose-limiting anemia developed. The patient failed to respond to cefadroxil, erythromycin, methotrexate, and azathioprine. Cyclosporine 4 mg/kg induced remission, but was cost prohibitive. Thalidomide 3 mg/kg was started with resolution of disease in 1 month. The thalidomide had to be withdrawn after 1 year of therapy because of the development of leg pain. This patient continues to have disease as an adult.
Intravenous immunoglobulin (IVIG) was reported in a 3-year-old boy with CBDC that initially responded well to dapsone and oral corticosteroids, but due to the development of diabetic ketoacidosis other treatments were sought [35]. His disease was difficult to control despite dapsone 2.5 mg/kg. Erythromycin was not helpful. IVIG 1 mg/kg was given monthly with a reduction in blistering and corticosteroid requirements. He remained clear except for episodic exacerbations during the next year.
Evaluation and Treatment Algorithm
CBDC is a “benign” condition in that it is not fatal and generally resolves spontaneously over a period of months to years with limited sequelae; however it can cause significant morbidity and usually requires systemic treatment to control the disease until spontaneous remission occurs. The goal of therapy in children is to suppress blistering and control pruritus with therapies that minimize the potential for serious adverse effects.
If a child presents with suspected CBDC it is important to obtain a biopsy from involved skin for routine histology along with a biopsy from perilesional skin for DIF. A thorough medication history should be obtained in all cases to rule out the possibility of drug-induced CBDC. The ocular and oral mucosae should be assessed carefully for evidence of involvement and the presence of scarring, which would necessitate more aggressive therapy. If ocular symptoms (pain, discharge, grittiness, redness) are present the child should be referred to an ophthalmologist. If nasal symptoms or hoarseness are present the child should be referred to an otolaryngologist.
There are no grading systems for the assessment of severity so the clinician must use his or her own judgment. Mild disease presents with a few, small localized blisters, often around the mouth and perineum, minimal symptoms and absence of mucosal involvement. Moderate disease probably describes the majority of children and presents with more generalized involvement with vesicles and bullae distributed over the face, trunk, perineum and extremities, mild to moderate pruritus, with or without non-scarring mucosal involvement. Severe disease presents with extensive, generalized involvement, severe and uncontrolled pruritus, and/or symptomatic mucosal involvement, with or without scarring.
Mild disease can often be managed with topical therapy, either a mid to high potency topical corticosteroid or tacrolimus ointment. If topical therapy is ineffective at controlling disease, a macrolide antibiotic (e.g., erythromycin) or semisynthetic penicillin (e.g., dicloxacillin) can be added. The antibiotics and dosing regimens are reported in Table 12.1. If the patient fails to respond to these conservative therapies, dapsone or sulfapyridine can be initiated.
Table 12.1
Summary of the antibiotics used to treat pediatric autoimmune bullous diseases
Antibiotic | Pediatric dosing | Side effects | Laboratory monitoring |
|---|---|---|---|
Dicloxacillin | a25–50 mg/kg/day | GI disturbances, allergic reactions, serum-sickness-like reactions, agranulocytosis, leukopenia, thrombocytopenia, hepatitis, nephritis | bPeriodic CBC, liver and renal function necessary for prolonged therapy |
Oxacillin | 50 mg/kg/day | GI disturbances, allergic reactions, serum-sickness-like reactions, agranulocytosis, leukopenia, thrombocytopenia, hepatitis, nephritis | Periodic CBC, liver and renal function necessary for prolonged therapy |
Erythromycin | 30–50 mg/kg/day | GI disturbances, QT prolongation, arrhythmia, rash, hepatitis | None recommended |
Trimethoprim-sulfamethoxazole | 8 mg/kg/d trimethoprim + 40 mg/kg/day sulfamethoxazole | Rash, SJS/TEN, GI disturbances, agranulocytosis, hemolytic anemia, hepatitis, nephritis |
First-line treatment for moderate to severe disease is either dapsone or sulfapyridine. Baseline investigations before starting either treatment include a screening test for the presence of G6PD deficiency, CBC, and liver and renal function tests. Dapsone is usually initiated at a dose of 0.5 mg/kg and titrated up to 2 mg/kg or until disease control is achieved. CBC is checked weekly for the first month, monthly for the next 5 months, then every 6 months. Liver function tests and reticulocyte counts should be monitored monthly for the first 6 months, then every 6 months. Methemoglobin levels should be checked if signs of methemoglobinemia (headache, fatigue, shortness of breath, cyanosis) occur. The response to treatment is generally rapid and response should be seen within 3 weeks. If the disease is not adequately controlled after 3 weeks, adjunctive therapies (most commonly systemic corticosteroids) should be added. Dapsone should be continued at the lowest possible dose as long as necessary to control disease. Periodic treatment withdrawal is necessary to determine if spontaneous remission has occurred. Dose dependent side effects include hemolysis and methemoglobinemia. Idiosyncratic adverse reactions include agranulocytosis, hepatitis, peripheral motor neuropathy, Steven-Johnson syndrome/toxic epidermal necrolysis, and dapsone hypersensitivity syndrome. Dapsone should not be used in patients with G6PD deficiency, severe anemia or hemolysis, or known sensitivity to sulfones or sulfonamides. Dapsone can be compounded in a 2 mg/ml oral suspension that is stable for 90 days; alternatively the 25 mg or 100 mg tablets can be crushed and mixed with jam or honey.
Sulfapyridine is no longer available in the United States, but is available in other countries. It is used by some as an alternative first-line treatment instead of dapsone. It can be combined with dapsone for improved efficacy without additive side effects [36]. The recommended dosing is 60–150 mg/kg and the reported dosing regimens vary from 250 mg to 3 g daily [16, 37]. Adverse effects of sulfapyridine are similar to dapsone, with a lower incidence of hematological side effects, but a higher incidence of cutaneous allergic complications [23].
If the disease is severe or dapsone or sulfapyridine monotherapy is not sufficient to suppress disease activity within 3 weeks, systemic corticosteroids should be added. Doses ranging from 0.5 to 1 mg/kg (sometimes up to 2 mg/kg) are necessary. As soon as the disease is adequately controlled, the corticosteroid should be tapered slowly over 3–6 weeks. If the patient requires frequent courses of systemic corticosteroids, is unable to be tapered off without repeated relapses, or develops unacceptable adverse effects alternative steroid-sparing agents should be sought. Alternative adjunctive treatments include antibiotics (Table 12.1), nicotinamide, mycophenolate mofetil, azathioprine, cyclosporine, intravenous immunoglobulin, or thalidomide (roughly in that order, but individualized to patient and disease severity).
In patients with G6PD deficiency alternative first-line therapies are colchicine and antibiotics. Colchicine can be used as monotherapy or in combination with corticosteroids. The recommended dose is 0.5 or 0.6 mg twice a day (only the 0.6 mg tablets are available in the US). Response should be seen within 2–6 weeks. Once disease control is obtained colchicine should be tapered to the lowest dose that controls disease. Baseline investigation includes CBC, liver and renal function, and urinalysis. These should be repeated periodically. Gastrointestinal disturbance (diarrhea, nausea, vomiting, abdominal pain) is the most common side effect and is dose dependent. Less common side effects include anemia, bone marrow suppression and peripheral nephropathy. Colchicine should not be used in patients with hepatic or renal impairment. See Table 12.2 for summary of treatment algorithm.
Table 12.2
Treatment algorithm for pediatric autoimmune bullous diseases
Disease | Severity | First line treatment | Second line treatment | Third line treatment |
|---|---|---|---|---|
Chronic bullous disease of childhood | Mild | Moderate to high potency topical CS or tacrolimus ointment | Antibiotic (see Table 12.1), dapsone (0.5–2 mg/kg) | Colchicine (0.6 mg BID), nicotinamide (30 mg/kg) |
Mod/Severe | Dapsone (0.5–2 mg/kg) or sulfapyridine (60–150 mg/kg) | Oral CSa (0.5–2 mg/kg) | Mycophenolate mofetil (35 mg/kg), azathioprine (2 mg/kg), cyclosporine (3–5 mg/kg), IVIG (2 g/kg q 2–4 weeks), thalidomide | |
Dermatitis herpetiformis | Gluten free diet and dapsone (0.5–2 mg/kg) | Sulfapyridine (60–150 mg/kg) or sulfasalazine | ||
Bullous pemphigoid | Mild | Moderate to high potency topical CS | Oral CS (0.5–2 mg/kg) or dapsone (0.5–2 mg/kg) | |
Mod/Severe | Oral CS (0.5–2 mg/kg) | Add dapsone (0.5–2 mg/kg) or sulfapyridine (60–150 mg/kg) | Mycophenolate mofetil (35 mg/kg), azathioprine (2 mg/kg), cyclosporine (3–5 mg/kg), methotrexate (0.3–0.5 mg/kg), rituximab (375 mg/m2 q week × 2–4 doses), IVIG (2 g/kg q 2–4 weeks) | |
Epidermolysis bullosa acquisita | Oral CS (1–2 mg/kg) and dapsone (1–2 mg/kg) | Mycophenolate mofetil (35 mg/kg) | Azathioprine (2 mg/kg), cyclosporine (3–5 mg/kg), methotrexate (0.3–0.5 mg/kg), IVIG (2 mg/kg q 2–4 weeks) | |
Pemphigus vulgaris | Mild | Moderate to high potency topical CSb | Oral CS (1–2 mg/kg) | |
Mod/Severe | Oral CS (2–3 mg/kg) | Azathioprine (2 mg/kg) or mycophenolate mofetil (35 mg/kg) or Methotrexate (0.3–0.5 mg/kg) | cRituximab (375 mg/m2 q week × 2–4 doses), IVIG (2 mg/kg q 2–4 weeks), cyclosporine (3–5 mg/kg), cyclophosphamide | |
Pemphigus foliaceous | Mild | Moderate to high potency topical CS | Oral CS (1–2 mg/kg), dapsone (0.5–2 mg/kg), hydroxychloroquine (3–5 mg/kg) | |
Mod/Severe | Oral CS (1–2 mg/kg) | Azathioprine (2 mg/kg), mycophenolate mofetil (35 mg/kg), Methotrexate (0.3–0.5 mg/kg) | cRituximab (375 mg/m2 q week × 2–4 doses), IVIG (2 mg/kg q 2–4 weeks), cyclosporine (3–5 mg/kg), cyclophosphamide |
Dermatitis Herpetiformis
Introduction
Like CBDC, dermatitis herpetiformis (DH) is an IgA-mediated disease that in certain populations may be more common than CBDC. DH is rare before puberty, but in one analysis of childhood onset the mean age was 7.5 years [15]. DH has been reported to occur as young as 8 months of age [38]. DH is more common in Europe and unusual in Africa and Asia [39]. Without treatment, the disease usually persists indefinitely. All children with DH have an underlying gluten-sensitive enteropathy.
Clinical Presentation
The disease classically presents with symmetrically-distributed, intensely pruritic, grouped urticarial papules and vesicles; due to the intense pruritus erosions and excoriations may be the most abundant sign. DH most frequently involves the elbows, knees, scapulae, shoulders, sacrum, buttocks, hairline and scalp. Acral petechiae or purpura have been reported more frequently in children [40]. The mucous membranes are generally spared.
Diagnosis
Histology of an intact vesicle shows subepidermal clefting with a predominantly neutrophilic infiltrate and dermal papillary microabscesses. DIF from perilesional skin shows the presence of a granular deposition of IgA within the dermal papillae, which is diagnostic for DH.
Treatments
Gluten-Free Diet
A gluten-free diet is the treatment of choice for DH because it improves both the cutaneous and gastrointestinal pathology. A review by Ermacora et al. [41] evaluated 76 children treated with a gluten-free diet alone. Cutaneous lesions resolved in 82 % of the children within 1–3 months. Because the onset of action is somewhat slow and strict adherence to the diet may be difficult, dapsone is usually used in conjunction with a gluten-free diet, at least initially.
Dapsone
Dapsone is FDA approved for the treatment of DH in pediatric patients (lower age limit not specified). The standard starting dose is 0.5 mg/kg increasing to 2 mg/kg until disease control is achieved. Although there are no controlled trials documenting the efficacy of dapsone as a treatment of childhood DH there is convincing evidence in the literature to support its use. Marsden [15] reported 25 children with DH; 15 were treated with dapsone and all 15 had rapid improvement in pruritus and skin lesions, often within 48 h. The monitoring and side effects of dapsone are discussed under CBDC.
Sulfapyridine
Sulfapyridine is an option, although less effective, in patients who can’t tolerate dapsone [15]. Sulfapyridine is no longer available in the US, having been replaced by its prodrug, sulfasalazine. Sulfasalazine is metabolized into sulfapyridine and is used in the treatment of inflammatory bowel disease. There are a few patients with suspected DH, including adolescents that have responded to treatment with sulfasalazine [42, 43].
Evaluation and Treatment Algorithm
The diagnosis of suspected DH in children should be confirmed with histology and DIF. If confirmed, referral to a gastroenterologist is warranted. A gluten-free diet is the treatment of choice and should be attempted in all patients. Dapsone is indicated for the rapid relief of pruritus and treatment of skin lesions. Once the patient is on a gluten free diet and skin lesions are controlled, dapsone should be tapered gradually to the lowest possible dose that controls the disease. In patients compliant with a gluten-free diet it can eventually be discontinued. If the patient is not controlled or cannot adhere to a gluten-free diet and is intolerant of dapsone, sulfapyridine or sulfasalazine can be tried. Moderate to high potency topical steroids can be used adjunctively on localized lesions.
Childhood Bullous Pemphigoid
Introduction
Bullous pemphigoid (BP) is another one of the more common immunobullous disease to occur in children. According to the cases reported in the literature it is much more common in Caucasians (61 % white versus 17 % black, 17 % Asian, 6 % Hispanic) and slightly more common in females (60 % of reported cases) [44]. There are two peaks of incidence in childhood BP: before age one (infantile BP) and again at 8 years of age [45]. A subtype of BP in children has been labeled localized vulval pemphigoid [46, 47]. Childhood BP has a very good prognosis, with remission occurring in most patients several weeks to a year after onset, with infrequent relapses. Despite the good prognosis, generalized disease can be life-threatening and should be treated appropriately [48, 49]. Childhood bullous pemphigoid has been reported to occur a few hours to 3 weeks after vaccination with DPT/polio alone or in conjunction with vaccines against pneumococcus, H. influenza, hepatitis B, or meningococcus. There are three cases that recurred with subsequent vaccinations, although the recurrence was less severe than the initial outbreak [50].
Clinical Presentation
BP presents with the abrupt onset of large, tense bullae on normal-appearing, red or urticarial skin, frequently accompanied by erosions and crusting (Fig. 12.3). Infantile BP more commonly involves the palms, soles, and face while childhood BP more commonly involves the genital region. Localized vulval pemphigoid involves only the vulva or scrotum [46, 47]. Mucous membranes are involved in approximately half of patients [51]. The blisters can be localized or disseminated. Pruritus is frequently present. The lesions heal without scarring.
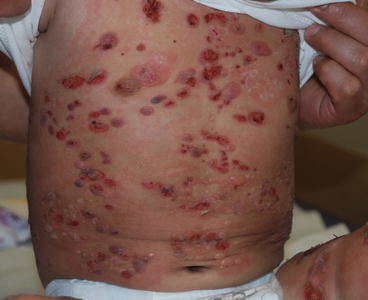

Fig. 12.3
Tense blisters, erythema and erosions on the trunk of a child with bullous pemphigoid
Diagnosis
Nemeth et al. [52] proposed the following criteria for diagnosis: (1) onset prior to 18 years of age; (2) tense blisters with typical histology (subepidermal blister with eosinophils); (3) DIF from perilesional skin showing a linear deposition of IgG and/or C3 as the major immunoreactant at the BMZ or a positive IIF showing circulating IgG antibodies directed against the BMZ. IIF on salt split skin reveals binding of IgG autoantibodies to the epidermal side and can be used to differentiate BP from epidermolysis bullosa acquisita (EBA); although rare cases with epidermal and dermal as well as pure dermal patterns have been reported [53]. Immunoblot assay or ELISA reveals circulating IgG autoantibodies directed against BP180/230 and can be useful for confirmation of diagnosis in uncertain cases. Eosinophilia is a frequent finding on CBC and may provide an early clue to diagnosis [54].
Treatments
Corticosteroids
Corticosteroids, systemic and/or topical, are the preferred treatment for childhood BP. Most cases reported have responded to moderate doses of systemic corticosteroids (0.5–2 mg/kg) that are able to be tapered off after a period of time without relapses. Topical corticosteroids have shown similar efficacy for localized disease. Waisbourd-Zinman et al. [45] described seven infants with BP. All infants were treated with prednisone 1–2 mg/kg and five infants responded with resolution of blisters within a few days to 3 months. They also summarized 71 additional cases from the literature. Twenty-five infants and children were treated with only systemic corticosteroids and almost all were reported to have a rapid response. Thirteen children were treated with only topical corticosteroids (ranging from low to high potency) with a varying rate of response. These children with localized disease, with the exception of one, had disease either localized to the palms and soles or vulva. Nineteen patients were not adequately controlled on systemic corticosteroids alone and adjunctive therapy with dapsone, sulfapyridine, IVIG, mycophenolate mofetil and/or cyclosporine was required. Intravenous pulsed methylprednisolone has also been reported to be effective at inducing remission [55].
Dapsone or Sulfapyridine
Dapsone and sulfapyridine have both been reported to be effective either as monotherapy or in combination with other medications, most commonly systemic corticosteroids. Marsden et al. [17] reported five patients (3 months to 14 years) with disseminated BP. Two patients were treated with dapsone (50–100 mg) and one patient was treated with sulfapyridine with a rapid response. Motegi et al. [56] reported a 14 year old girl with BP localized to hands and feet who was successfully treated with dapsone. Treatment with dapsone 1.5 mg/kg resulted in complete healing in 1 month and remission by 1 year. However, there have been several other reports that have not substantiated the beneficial effect of sulfones alone for BP in children [52].
Dapsone and sulfapyridine are more commonly used as adjuvant therapies for patients with generalized disease. There are numerous reports in the literature documenting the use of dapsone or sulfapyridine in conjunction with corticosteroids, but details are limited [45]. Petronius et al. [57] reported a 3.5 month old female with generalized BP that did not respond adequately to methylprednisolone 0.75 mg/kg. The addition of dapsone 1 mg/kg titrating up to 4 mg/kg resulted in a complete response within a few weeks. There are reports of two infants and a 3-year-old with generalized BP showing a complete and rapid response to corticosteroids and dapsone [55, 58]. It is difficult to interpret what effect the dapsone had on the response to treatment because the patients were treated with both medications simultaneously. Dapsone has also been used in combination with erythromycin and niacinamide [59].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






