8 Artefill®
a third-generation polymethylmethacrylate in collagen soft tissue filler
Summary and Key Features
• Artefill® is the newest generation polymethylmethacrylate (PMMA) filler
• Artefill® consists of PMMA microspheres suspended in bovine collagen
• The PMMA microspheres of Artefill® are more uniform in size than the earlier generation PMMA fillers and there are very few particles less than 20 µm, thereby preventing phagocytosis and subsequent granuloma formation
• Artefill® is best for deeper creases and folds rather than fine lines
• In the USA, the Food and Drug Administration (FDA) indication is for nasolabial folds
• Artefill® lasts at least 5 years and may be permanent in some patients
• Placement should be in the deep dermis or at the dermal–subcutaneous junction
• Skin testing to the bovine collagen component of the filler should be performed at least 1 month prior to treatment with Artefill®
• Although rare, granuloma formation may occur years after injection of Artefill®
• Different types of anesthesia may be used prior to Artefill® placement
Introduction
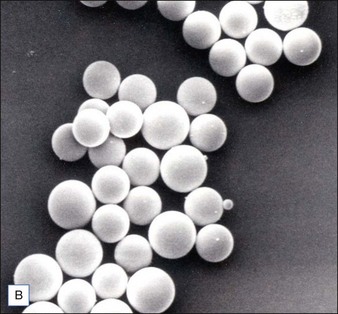
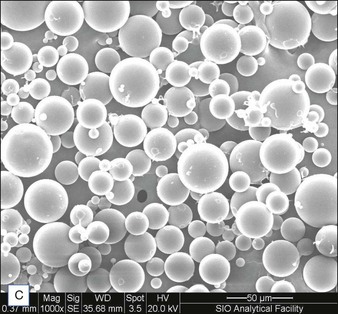
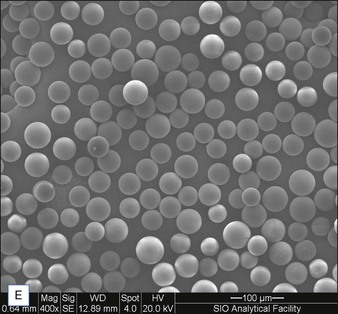
Biological dermal fillers reliably and safely augment facial wrinkles and folds, but necessitate repeat treatments after they have been resorbed, generally within a 12-month period. Artefill®, a novel permanent implant, was developed to overcome this limitation. This soft tissue filler is composed of polymethylmethacrylate (PMMA) microspheres suspended in a collagen gel matrix containing 0.3% lidocaine. It is a third-generation PMMA-based filler product that contains an optimized collagen matrix with PMMA microspheres that have enhanced uniformity and consistency as well as near elimination of nanoparticles. It is critical to distinguish Artefill® from prior generation PMMA products (Artecoll® and Arteplast®). Not all PMMA products are the same and scanning electron microscopy can show a wide variation of nanoparticles, sphere irregularities, and sphere shapes and sizes (Fig. 8.1), all of which might contribute to early or late adverse events.
Patient selection / treatment areas
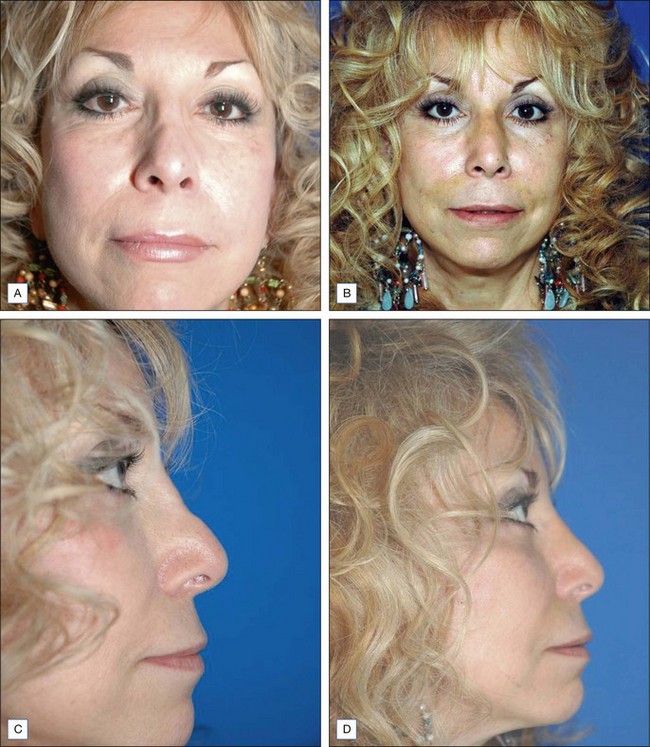
Some of the other potential off-label indications for Artefill® are in the permanent treatment of selected primary and secondary nasal deformities and for dorsal nasal augmentation (Fig. 8.2). Permanent reconstruction of lateral chin contour abnormalities following genioplasty, small depression defects about the head and neck, permanent nipple augmentation and acne scar injection are other off-label indications that might benefit from Artefill®.