Lasers have a rich history in ophthalmology
Lasers have been used to treat common diseases such as diabetic retinopathy and macular degeneration as well as rare conditions such as intraocular tumors
Several types of lasers are used in the posterior segment including argon, diode, and photodynamic therapy
Lasers are commonly used in the clinic setting and operating room
Introduction and History
Among medical fields, ophthalmology has perhaps the richest history with regard to the widespread application of laser technologies. The first experimental use of laser in ophthalmology was that of the German ophthalmologist Gerd Meyer-Schwickerath, who began using the Beck arc in 1949.1,2 By 1954, Meyer-Schwickerath had treated 41 patients with the xenon arc photocoagulator and by 1957, he reported that he was able to close 82 macular holes with this technology.1 Working together with Littmann from the Carl Zeiss Company, he created a similar xenon arc photocoagulator which became available for widespread ophthalmic applications in the late 1960s and was used more frequently in the 1970s. Since then, lasers have been used with notable success for a wide variety of ophthalmic conditions including refractive error, glaucoma, lens-related conditions such as posterior capsular opacification, and retinal conditions including diabetic retinopathy and age-related macular degeneration.
This chapter will outline the past and current uses for laser in the posterior segment of the eye. Anatomically speaking, the term posterior segment generally refers to the vitreous, retina, choroid, and posterior sclera. As such, the laser procedures discussed herein are most commonly performed by retina specialists in subspecialized medical settings. Table 1 summarizes current posterior segment laser applications. In many cases, similar procedures have been adapted for varying ophthalmic conditions.
Table 1
Major current posterior segment applications for laser in ophthalmology
Laser type | Procedure name | Wavelength (nm) | Disease/indication |
|---|---|---|---|
Argon/krypton/dye | Pan retinal photocoagulation | 488–647 | • Proliferative diabetic retinopathy |
Argon/krypton/dye | Focal laser photocoagulation | 488–647 | • Diabetic macular edema |
Argon/krypton/dye | Photocoagulation of subretinal neovascular membrane/ complex | 488–647 | • Age-related macular degeneration |
• Many other causes including: infections, inflammatory conditions, idiopathic central serous chorioretinopathy, retinal or choroidal tumors | |||
Argon/krypton/dye | Retinopexy for retinal tear | 488–647 | Retinal tear demarcation to prevent retinal detachment |
Photodynamic therapy (PDT) | PDT | 689 | • Age-related macular degeneration |
• Subretinal neovascular membranes due to infections or inflammatory conditions | |||
• Idiopathic central serous chorioretinopathy | |||
• Retinal or choroidal tumors | |||
Diode | Transpupillary thermotherapy (TTT) or diode therapy | 810 | • Ocular tumors such as retinoblastoma or melanoma |
Endolaser for vitreoretinal surgery (argon green or diode) | Pan-retinal photocoagulation | varied | • Proliferative diabetic retinopathy |
Argon Laser
The three current common procedures for which argon laser is used are: pan-retinal photocoagulation (PRP), focal macular coagulation, and photocoagulation of choroidal or subretinal neovascular membranes or complex, and lasering of retinal holes or tears. The current treatment recommendations which incorporate the DRS findings and those of later large scale trials include: high risk proliferative diabetic retinopathy. The complications of PRP in the DRS were generally mild and included a decrease in visual acuity of 1 or more lines in 11% and peripheral visual field loss in 5%. |
The three current procedures for which argon laser is used are: pan-retinal photocoagulation (PRP), focal macular coagulation, and photocoagulation of choroidal or subretinal neovascular membranes or complexes.
Indications
Pan-Retinal Photocoagulation
Full scatter PRP is a treatment approach which was first established on a widespread basis in the 1970s in the Diabetic Retinopathy Study (DRS) 3 and then further explored in the 1980s and early 1990s in the Early Treatment Diabetic Retinopathy Study (ETDRS).4 The theory behind PRP for proliferative diabetic retinopathy (PDR) is that by destroying the peripheral retina, it decreases the stimulus for the growth of new abnormal blood vessels. The DRS enrolled 1,742 patients, 876 of whom were randomized to the argon group and 875 to the xenon group.5 In the end, the harmful effects of xenon coagulation were more significant than for argon, thus argon laser became the standard of care.
The current treatment recommendations which incorporate the DRS findings and those of later large scale trials include: high risk proliferative diabetic retinopathy which is defined as: mild neovascularization of the optic disc (NVD) with vitreous hemorrhage, moderate to severe NVD with or without vitreous hemorrhage, or moderate neovascularization elsewhere in the retina (NVE) with vitreous hemorrhage (Figs. 1a–b and 2). High risk proliferative diabetic retinopathy is also defined in any case with three of the following four risk factors: vitreous or preretinal hemorrhage, presence of new vessels, location of new vessels on or near the optic disc, and moderate to severe extent of new vessels. In the DRS, the risk of severe visual loss (defined as a visual acuity of less than 5/200) was 26% for patients with high risk proliferative diabetic retinopathy versus 7% in patients without the aforementioned high risk characteristics after 2 years.6 PRP reduced this risk of severe visual loss by 50%. In addition to these criteria, many studies including ETDRS have suggested that PRP may be indicated for patients with severe nonproliferative diabetic retinopathy in special high risk situations such as poor compliance history, impending pregnancy, or impending cataract surgery.4
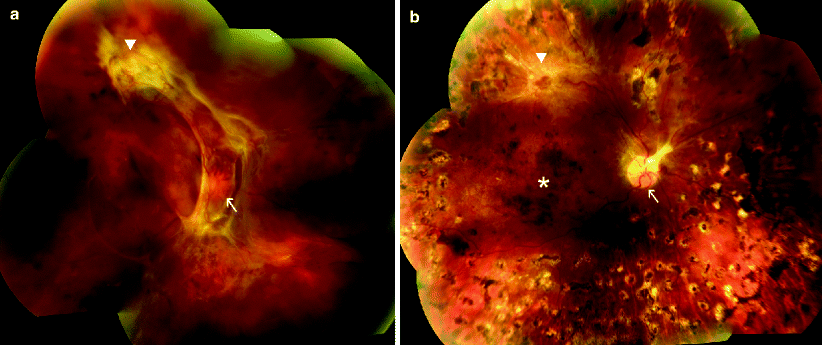
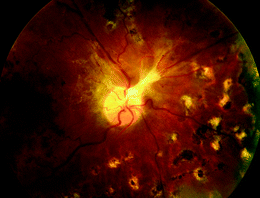

Fig. 1
59-year-old female with proliferative diabetic retinopathy, fundus photographs. (a) Extensive neovascularization of the optic nerve head (arrow) and along the superior macular vascular arcade (triangle) with associated fibrosis and retinal traction. (b) Five months after pan-retinal argon laser photocoagulation. Neovascular complexes involving the optic nerve head (arrow) and along the superior macular arcade (triangle) have regressed, with residual fibrosis. Foci of atrophic retina with pigment clumping are seen throughout the periphery at sites of laser treatment. Multiple, confluent dot blot hemorrhages are seen in the macula (star)

Fig. 2
59-year-old female with proliferative diabetic retinopathy, fundus photograph 5 months after pan-retinal argon laser photocoagulation. Fibrotic scar involving the optic nerve head is seen following regression of the neovascular complex. Foci of atrophic retina with pigment clumping are seen at sites of laser treatment. These foci are noted approximately one disc diameter from the optic disc and extend into the periphery
Focal Argon Laser Photocoagulation
Macular edema is responsible for a major part of visual loss in diabetic retinopathy. Many of the current treatment paradigms are based on the results of the ETDRS.4 The study enrolled 3,711 patients between 1980 and 1985 who had either (a) no macular edema with visual acuity better than 20/40 or (b) macular edema with visual acuity better than 20/200. Clinically significant macular edema (CSME) was defined in the study as one of the following: thickening of the retina at or within 500 μm of the center of the macula, hard exudates at or within 500 μm of the center of the macula if associated with thickening of the adjacent retina, or a zone of retinal thickening of greater than or equal to 1 disc area within 1 disc diameter of the center of the macula (Figs. 3a–c and 4a–b). The ETDRS results demonstrated that eyes with CSME benefited from focal laser photocoagulation by reducing the risk of moderate visual loss by at least 50% and increasing the chance of visual improvement.7 This effect was maintained over time with moderate visual loss at 3 years of follow-up in 24% of treated patients treated versus 12% of untreated patients. The study concluded that patients with CSME and good vision should be considered for treatment based on other factors such as status of the fellow eye, anticipated cataract surgery, proximity of exudates to the fovea, or the presence of high-risk PDR.8
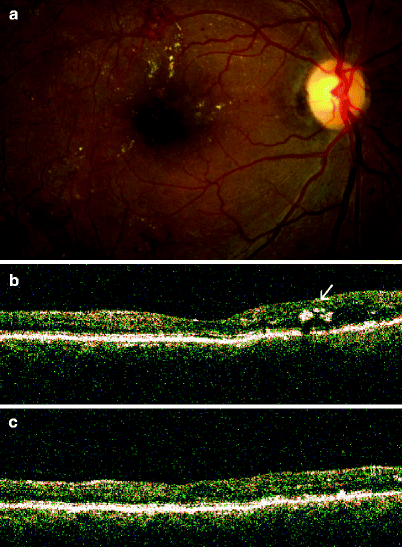
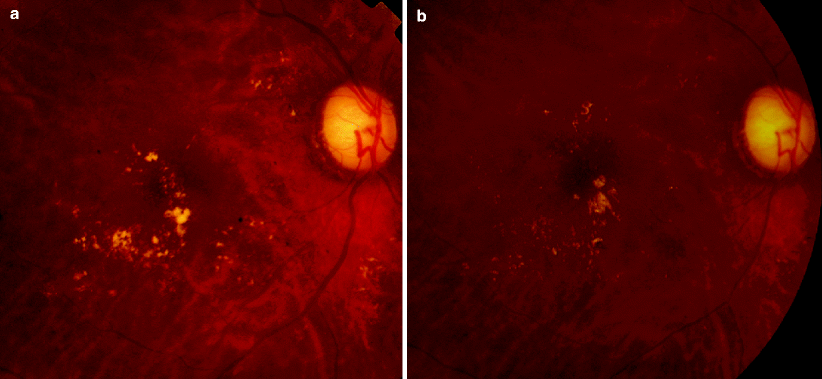

Fig. 3
54-year-old man with diabetic macular edema treated with focal argon laser. (a) Fundus photograph: clinically significant macular edema in the right eye. Yellow, refractile, hard exudates superiorly indicate an area of retinal thickening. (b) Optical coherence tomography (OCT): vertically oriented scan with the retinal area located inferior to the fovea represented on the left and the retinal area located superior to the fovea represented on the right. The superior retina is thick compared to the inferior retina because of diabetic macular edema. Intraretinal hard exudates (arrow) are seen in the superior retina as manifested by shadowing, with diminished detection of the more posterior retinal layers. (c) OCT of the same retinal area as shown in (b): 3 months after focal argon laser treatment. The superior retina is no longer thick and there has been resolution of the intraretinal hard exudates

Fig. 4
60-year-old female with diabetic macular edema treated with focal argon laser. (a) Fundus photograph: clinically significant macular edema in the right eye. Yellow, hard exudates indicate areas of retinal thickening. (b) Fundus photograph: marked resolution of hard exudates 5 months after focal argon laser treatment
Laser for Choroidal Neovascular Membranes in Neovascular ARMD and Other Conditions
For many years, laser photocoagulation was the only proven treatment for choroidal neovascularization associated with neovascular macular degeneration and other retinal conditions. In the late 1980s and early 1990s, the Macular Photocoagulation Study (MPS), a series of eight multicenter, randomized, prospective trials examining the use of argon and krypton laser for choroidal neovascular membranes was published.9 This study evaluated the use of laser for extrafoveal and juxtafoveal lesions in three conditions: neovascular ARMD, presumed ocular histoplasmosis (POHS), and idiopathic choroidal neovascularization. One major drawback of these trials was the narrow eligibility criteria: no more than 15–20% of neovascular ARMD cases present with well-defined choroidal neovacularization as required by the trial.
In general, in all of the MPS trials, treatment did not decrease the patient’s chance of maintaining stable visual acuity, but the proportion of eyes, treated or untreated, that maintained good or stable visual acuity was very small. The reason for this inadequate treatment effect is that despite treatment, many eyes continued to lose vision because of persistent or recurrent neovascularization that extended into the foveal center. Subfoveal recurrences were not treated with laser in these trials due to concerns about permanent central visual loss. Due to its deleterious effect on normal surrounding neural retina, the use of argon laser for choroidal neovascularization near the fovea sharply decreased with the advent of photodynamic therapy (see below) and anti-vascular endothelial growth factor therapies. However, argon laser does continue to have a rarely used but important role in that it is highly effective for extrafoveal isolated choroidal neovascular lesions. Patients should always be informed that this treatment induces a permanent scotoma.
Technique
Pan-Retinal Photocoagulation
Argon laser can be applied for PRP from a slit-lamp based or indirect ophthalmoscopic system (Fig. 5). The slit lamp-based system is used for a seated patient and consists of a modified slit-lamp (specialized microscope for ophthalmoscopic exam) mounted on a table. The retina is visualized by using a wide-field contact lens (Fig. 6). The indirect system is composed of a headset worn by the ophthalmologist which emits the laser directly. For this system, the patient is typically reclined in an examining chair and the retina is visualized by using a 20-Diopter or 28-Diopter lens (Fig. 7).
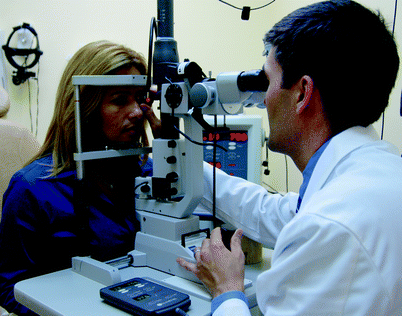

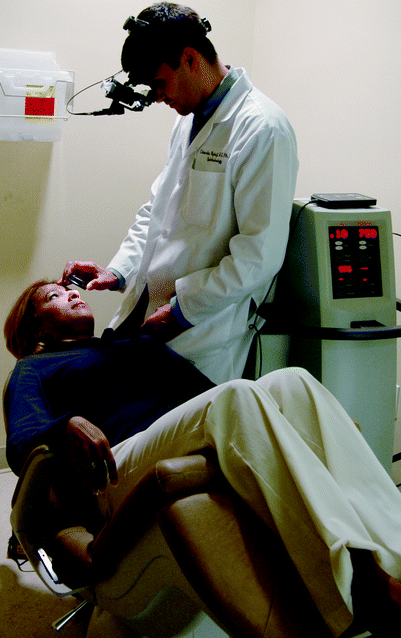

Fig. 5
Slit-lamp based delivery of argon laser photocoagulation. The patient is seen seated to the left with her face firmly placed in an ophthalmic microscope modified for laser delivery. The ophthalmologist views the retina and applies argon laser burns through a corneal contact lens seen in the physician’s right hand

Fig. 6
Commonly employed ophthalmic lenses. A multitude of lenses can be used for argon laser treatment application depending on the desired magnification and field of view. Such lenses can be broadly categorized as either corneal contact lenses (five lenses located on the left: ocular Mainster 165, ocular three mirror, ocular Yannuzzi fundus, ocular Reichel-Mainster and ocular Mainster wide field) or indirect lenses (two lenses located on the right: 20-diopter and 28-diopter)

Fig. 7
Indirect-ophthalmoscopic based delivery of argon laser photocoagulation. The patient is seen reclined in an examining chair. The ophthalmologist applies argon laser spots using a headset-based laser and visualizes the retina using either a 20-diopter or a 28-diopter lens seen in the physician’s right hand
The standard technique for PRP currently involves the placement of 800–1,600 laser burns with a 500 μm spot size, spaced 0.5 burn widths apart from each other with 0.1–0.2 s of duration.10 Intensity is regulated so that mild white bleaching is obtained (Fig. 8). The treatment reaches from the temporal arcade to the equator, and up to 2 disc diameters temporal to the macular center. Typically one disc diameter or space is spared around the optic nerve to avoid central visual field defects.
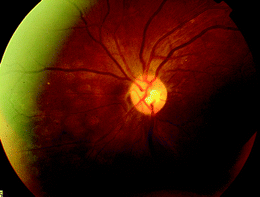

Fig. 8
42-year-old female with proliferative diabetic retinopathy underwent pan-retinal argon laser photocoagulation. Fundus photograph of retinal burns 3 h after application. Discrete, cream-colored round lesions are seen deep to the retinal vasculature. Over the ensuing weeks to months, these subtle lesions will evolve into more noticeable areas of retinal atrophy, often with associated pigment clumping, as seen in Figs. 1b and 2
Focal Argon Laser Photocoagulation
Focal laser is typically applied using a slit-lamp based system for a seated patient (Fig. 5). A magnifying contact lens is held by the ophthalmologist for detailed viewing of small retinal features (Fig. 6). Focal laser treatment typically consists of 50–100 μm laser burns of 0.05–0.1 s duration applied to microaneurysms between 500 and 3,000 μm from the center of the macula with the clinical endpoint defined as a color change to a mild whitening. For more diffuse macular edema, a grid pattern is typically applied in the following manner: 100–200 burns of a 50–200 μm spot size spaced 1 burn width apart within 2 disc areas of the fovea. In the ETDRS, an average of 3–4 treatment sessions 2–4 months apart were required.7 The grid technique has been demonstrated in several more recent studies to be more effective than milder focal techniques in reducing retinal thickening based on detailed measurements taken with the optical coherence tomography (OCT) and thus continues to be the standard of care.11
Laser for Choroidal Neovascular Membranes in Neovascular ARMD and Other Conditions
Laser for choroidal neovascular membranes is typically applied using a slit-lamp based system for a seated patient. A magnifying contact lens is held by the ophthalmologist for detailed viewing of small retinal features. In the MPS studies, argon laser was applied to cover the choroidal neovascular membrane (location judged on fluorescein angiogram) and 100 μm beyond the edge of the lesion, but was never applied closer than 200 μm from the center of the fovea. The laser was set initially at a 200 μm spot size, 0.2–0.5 s duration, and 100–200 mW power. The power was adjusted to achieve an intensity sufficient enough to produce a uniform whitening of the overlying retina.
Adverse Events
Pan-Retinal Photocoagulation
Focal Argon Laser Photocoagulation
Laser for Choroidal Neovascular Membranes in Neovascular ARMD and Other Conditions
Complications from argon laser for choroidal neovascularization include: hemorrhage, perforation of Bruch’s membrane, retinal pigment epithelial tear, and arteriolar narrowing.9,13




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








