Several non-invasive neoplastic and infectious conditions of the external female genitalia are amenable to treatment with a variety of ablative lasers.
Ablative laser treatment of the vulva, vagina and cervix provides a relatively fast treatment modality and results in healing with little scar formation and excellent cosmetic and functional results, as well as fairly uncomplicated postoperative recovery.
Intra-abdominal uses of different lasers are valuable alternatives to other thermal or mechanical cutting instruments.
Severe side effects and complications of laser use can be minimized by careful patient selection, using the most appropriate instruments, proper surgical technique, and meticulous postoperative care.
Good candidates for laser ablative procedures are generally considered to be individuals who have been refractory to medical and/or chemical treatment, and those presenting with extensive disease, as well as patients in whom a single surgical procedure is indicated for medical, psychological, or social reasons.
With ongoing advancements in laser technology and techniques, improved clinical outcomes with minimal postoperative recovery will be realized.
Introduction
Gynecologists first used laser in 1979 and have used CO2, KTP-532, Argon, and Nd:YAG to treat lower genital tract, intrauterine, and intra-abdominal disease. |
The use of CO2 laser in the treatment of uterine cervical intraepithelial lesions is well established and indications, as well as techniques, have changed very little for over 30 years. The Cochrane Systematic Review from 2000 suggests no obviously superior technique. CO2 laser ablation of the vagina is also established as a safe treatment modality for VAIN (Vaginal Intraepithelial Neoplasia), and has been used extensively in the treatment of VIN (Vulvar Intraepithelial Neoplasia) and lower genital tract condylomata acuminata. CO2 laser permits treatment of lesions with excellent cosmetic and functional results. The treatment of heavy menstrual bleeding by destruction of the endometrial lining using various techniques, including Nd:YAG laser ablation, has been the subject of a 2002 Cochran Database Review. Among the compared treatment modalities are modified laser techniques. The conclusion by reviewers is that outcomes and complication profiles of newer techniques compare favorably with the gold standard of endometrial resection. Myoma coagulation or myolysis with Nd:YAG laser through the laparoscope or hysteroscope is a conservative treatment option for women who wish to preserve their child bearing potential. The ELITT diode laser system is one of the new successful additions to the European armamentarium. CO2 laser is the dominant laser type used with laparoscopy for ablation of endometriotic implants. The KTP-532 nm laser also has been used for essentially all of the previously mentioned applications of carbon dioxide. It is less widely available, but does offer certain distinct advantages which will be discussed further.1
History of Procedures
In 1973 laser was first used in gynecology by Kaplan for vaporization of infected cervical tissue. The use of the laser through a laparoscope was first described by Bruhat in 1979. Goldrath first described intrauterine procedures using the Nd:YAG in 1981. The media used in gynecologic surgeries are CO2 and argon gases, as well as KTP, Nd:YAG crystals, and diode lasers. |
Albert Einstein postulated his idea of stimulated emission of radiation in 1917,2 but it took 40 more years for this idea to be converted into a practical device. In 1958 Arthur L. Schawlow and Charles H. Townes published their initial article covering the basic principles of the laser in the American Physical Society’s Physical Review.3,4 This was followed by their first proposal of gas lasers excited by electrical discharge. In 1960 Ali Javan, William Bennett, and Donald Herriott constructed a helium neon laser, the first laser to generate a continuous beam of light.2 In 1961 the first continuous operation of an optically pumped solid-state laser Nd:CaWO4 by L.F. Johnson, G.D. Boyd, K. Nassau, and R.R. Soder was reported.2 C.K.N. Patel developed the first CO2 laser in 1964.5 The same year the Nd:YAG laser was introduced by J.F. Geusic and R.G. Smith.2 The first experimental medical application was reported in 1965.6
In 1973 laser was first used in gynecology by Kaplan for vaporization of infected cervical tissue.7 The following year Bellina reported the first definitive procedures done on the vulva, vagina and cervix using the CO2.8 Over the next decade, hundreds of articles were published discussing the techniques of the use of carbon dioxide laser and the treatment of intraepithelial neoplasia and condyloma of the lower genital tract. In 1989 Yandell presented information regarding excisional cone biopsy of the cervix using the argon, KTP-532, and the Nd:YAG lasers, touting marked improvement in hemostasis and application of the energy using the shorter wavelength fiber optic instrumentation.1
Intra-abdominal and intrauterine applications were also explored. Bruhat first described use of carbon dioxide layer through the laparoscope in 1979.9 Three years later, Keye reported on the use of argon laser for the treatment of endometriosis.10 This was followed very shortly by introduction of the KTP-532 and the Nd:YAG lasers laparoscopically. In 1981 Goldrath first described the use of Nd:YAG laser in the endometrial cavity for the destruction of the endometrium and later, for resection of the uterine septa, submucous myomata, and excision of polyps.11 In 1984, Rettenmaier first published data on the treatment of gynecologic tumors of the vagina and vulva using photoradiation with hematoporphyrin dyes.12
Epidemiology of Human Papillomaviruses
Human papilloma virus (HPV) infections, and genital HPV in particular, are serious public health concerns, not just due to their immediate impact on quality of life, but also due to the tremendous economic burden to the affected patient and the public.13 In the USA, close to $3.5 billion are spent annually for the treatment of HPV-related conditions.
Classification of Virus Types
Human papilloma viruses only have affinity to the human body. Almost 200 different types have been identified to date. They are subcategorized according to tissue tropism: cutaneous versus mucosal, and oncogenic potential. Depending on the host’s immuno competence, these infections may be transient or persistent.14
Emphasis in this chapter will be placed on anogenital tract infections. There are about 10–15 low-risk types, with types 6 and 11 being most prominent, which are responsible for genital wart growth. There are 15–20 high risk or oncogenic types, which are responsible for precancerous and cancerous transformation of genital epithelial tissues. The most prevalent high risk types are 16, 18, 31, 33, 35, 39, 45, 51, and 52.15–18
Risk Factors
Genital HPV infections are predominately sexually transmitted. Vertical transmission from delivering mother to newborn is confirmed for respiratory papillomatosis.17 The risk for virus acquisition is directly correlated with the number of sexual partners.14,19–22 Smoking is an additional risk factor, as may be the use of contraceptive pills in women.23 Condom use appears to provide incomplete protection due to the involvement of uncovered genital contact sites.
Incidence and Prevalence
Estimates of the infection rate within populations are challenging because of the unpredictability of the natural history, the lack of requirements to report the disease, and the large variations between different populations and age groups. The overall risk of infection is ultimately related to sexual behavior and risk factors. HPV is considered the leading sexually transmitted infection in the USA. According to data from the Centers for Disease Control (CDC) and the National Health and Nutrition Examination Survey (NHANES), at least 50% of sexually active men and women will acquire HPV infections at some point in their lives.24 Not surprisingly, adolescents and young adults show the highest incidence numbers.21,25 HPV infections in men are less extensively studied, but infection rates appear similar to those found in women and are estimated to be as high as 73%.23,26 Male circumcision appears to decrease the risk of infection for the male and probably the risk of transmission of the virus.27
Studies on the distribution of different virus types within 11 countries from Africa, Europe, and South America demonstrated geographic variation, with the HPV 16 type being more prominent in Europe.28
Tissue Tropism and Clinical Infections
Several HPV types have a propensity to infect keratinizing epithelium and cause cutaneous warts, such as common warts and plantar warts (types 1,2,4), butcher’s warts (types 2 and 7), and flat warts (type 3 and 10).29,30 Bowen’s disease is a form of squamous cell carcinoma in situ from which numerous virus types have been isolated: 16, 18, 31, 32, 34 and others.18,29,31,32
Several of the above mentioned virus types also infect non-keratinizing epithelial surfaces, especially within the anogenital region, but also within the mouth and pharynx. Condylomata acuminata are the best known genital warts and affect at least 1% of the sexually active population, with the peak prevalence in the young adult age group.19,20,22,32 Subclinical infections are very common and constitute a major challenge for the treating physician. Colposcopy and acetic acid are required tools for detection of these latent stages.
There is now ample evidence that links persistence of high risk HPV types to the development of cervical cancer and other surface cancers of anal, vulvar, penile, and oropharyngeal origin.18,33–36 In the past, cervical cancer was the most frequent malignancy among women in developing countries, but it now ranks second after breast cancer.18
Outlook
The introduction of the quadrivalent papilloma virus vaccine for adolescent and young adult females in 2006 will positively impact the epidemiology of immunized women in the decades ahead, but generations of already infected women will need attention for many years to come.
Laser Use on Vulva, Vagina, and Cervix
Indications and Contraindications
Indications – Intraepithelial Neoplastic Disease – Condyloma acuminata refractory to medical and chemical treatment. – Cervical Stenosis – Extensive Disease including extension into anus and urethra/bladder of condyloma acuminatum. Contraindications – Patients in whom invasive cancer has not been ruled out. |
Since the instruments first became available to gynecologists, laser has been used in the treatment of pre-malignant (dyplastic) lesions of the lower genital tract. These include cervical intraepithelial neoplasia (CIN), vaginal intraepithelial neoplasia (VAIN), and vulvar intraepithelial neoplasia (VIN).
The vast majority of these intraepithelial neoplasias are of the uterine cervix, with the incidence rising dramatically over the last four decades. This increase parallels the rise in infection rates of human papilloma virus in the young female population. At birth, the squamo-columnar junction between the vagina and the endocervical columnar epithelium lies at the outer aspects of the ectocervix. At menarche, the vaginal pH drops substantially from 7.2 to 4.5. This, coupled with a marked effect on the vaginal flora, causes the onset of metaplastic change over the columnar epithelium that is exposed to the vaginal environment. During the course of metaplastic change, this exposed endocervical tissue is covered by a pseudo-stratified squamous epithelium with small infoldings in the surfaces down to the level of the original columnar tissue. These infoldings are frequently, and incorrectly, described as glands or crypts, for lack of a better term. The entire process takes approximately 8–10 years, and during this time the tissues of this transformation zone are extremely susceptible to the virus. Once the HPV is incorporated into the cells, they may undergo neoplastic transformation or simply remain infected, depending on the specific viral sub-type. The body may recognize the virus as foreign and mount an immune response, but in many cases, it does not, allowing persistent infection or neoplastic change, which can then advance in severity. The lesion spreads over the cervical surface and as it does so, it also moves down into these “glands” of the newly formed transformation zone.
When the patient presents, usually following an abnormal pap smear, the work up includes colposcopy with biopsy of the most suspicious areas, to determine the severity of the disease. Because the lesion is intraepithelial, destruction of the epidermis is adequate for treatment of the neoplastic lesion; however, it is known that large areas of the normal appearing transformation zone are infected by the virus despite no visible lesions being present on colposcopic exam. The other concern regarding treatment is that because of the infolding of the epithelium, the dysplastic lesion may extend several millimeters below the surface, and into the endocervical canal. With this in mind, the generally accepted treatment is destruction of the entire transformation zone to the depth of 5–7 mm.
Treatment methods used in the past were diathermy, and later cryotherapy. Neither of these modalities allows the physician to discern the depth of destruction at the time of the procedure. The use of laser, however, allows very accurate vaporization or ablation of the transformation zone down to the desired depth, with extension of that vaporization further up into the endocervical canal to visibly and measurably treat the entire extent of the tissue involved.
Evaluation and treatment of vaginal and vulvar intraepithelial neoplasia is similar, and in some ways simpler, because the epithelium involved is completely exposed, unlike that of the uterine cervix. Care must be taken, especially in the vagina, to not destroy more than the effected epithelium, which generally is less than 1 mm in thickness. Problems also arise in treating the portion of vulva in which there is hair because of the spread of the disease down into the follicles. Another concern with VIN is that it tends to be multifocal, requiring very careful colposcopic exams and frequently several biopsies in order to identify the extent of lesions.
Of paramount importance is insuring that there is no invasive disease prior to using local destructive treatments. Any suspicion of invasion requires further excisional tissue diagnosis. For many years, excisional cone biopsy was performed using the “cold” knife, or “hot” electrocautery. This is a markedly inaccurate procedure which removes the entire ectocervix and the distal and middle portion of the endocervical canal for tissue evaluation. The “Cold knife” cone is fraught with potential complications including excessive blood loss; inadequate incision depth, which may make it difficult to discern whether the lesion is invasive or microinvasive if it is incompletely excised; and excessive tissue removal, resulting in incompetent cervix and subsequent second trimester pregnancy loss. Because of their precision and hemostatic characteristics, lasers have been used for excisional cone biopsies by many surgeons for the last 20 years.
The other major indication for the use of laser of the lower genital tract is the treatment of condyloma accuminata. These lesions are generally first treated conservatively using cytotoxic agents such as podophylin, immune modulators such as imiquimod, or acids such as TCA for the destruction of specific early disease. Cryocautery may also be used for destruction. However, in most cases, each of these requires multiple treatments which can be fairly painful and irritating. The use of cryocautery may also be complicated by excessive destruction into the dermis, which causes scarring and may result in ulceration and infection. Because large areas of skin are infected with the virus and appear normal at the time of initial treatments, it is very common for secondary lesions to become apparent around lesions which have been previously treated. In some cases the local treatment itself may not be adequate to cause destruction of the primary lesions. Frequently patients present with extensive disease involving large areas of the lower genital tract and local treatment using medical or chemical means is simply impractical. These patients are generally treated primarily with laser in the operating room for the best results. In a significant number of these cases, the condylomata extend into the anal canal and may also extend into the urethra and bladder neck. The KTP-532 laser may be used inside the bladder and urethra for precise destruction of lesions in a fluid environment.
One known complication of conventional cone biopsy of the cervix is stenosis of the cervical os. In this situation, hypertrophic scarring of the surgical defect essentially closes the endocervical canal to the point that menstrual flow may be obstructed, or secondary infertility becomes an issue. The best treatment for this condition is CO2 vaporization of the scar tissue which has occluded the canal. Following this procedure, the endocervical columnar epithelium typically grows outward as the squamous tissue grows in from the exocervix, creating a more normal patent opening.
Techniques
Adequate preoperative patient evaluation and education. Timing of the procedure to closely follow the menstrual cycle. Mechanical bowel prep is indicated in the majority of cases. Antibiotics are rarely indicated if the appropriate depth of destruction is maintained. The Carbon Dioxide and KTP-532 lasers are both used for the treatment of lower tract disease. Although the CO2 laser is the most commonly used, the KTP-532 offers the important benefit of substantially less post-operative pain, which is the single most significant morbidity encountered. Care must be taken not to ablate too deeply, especially in the vagina and over opposing vulvar surfaces. Early postoperative evaluation is the key to avoiding major complications. |
Preoperative Management
There is no consensus among laser experts regarding the most appropriate preoperative regimen for laser use in gynecology. Adequate preoperative patient evaluation and education are paramount because of the relatively long and sometimes painful postoperative course, and the relatively high persistence and recurrence rates of both intraepithelial neoplasia and HPV. It is always best to time the procedure just after the menstrual period to allow the longest time possible for healing before the next menses. In some instances it is appropriate to postpone menstruation by using hormonal therapy such as injectable depomedroxyprogesterone or oral contraceptive suppression.
It is always advantageous to administer a mechanical bowel prep prior to any extensive procedure. This will decrease exposure of the surgical field to bowel flora. The prep should be administered the day before surgery, as enemas given on the same day tend to increase contamination during the procedure.
Due to the moist, de-epithelialized state of ablative laser-treated skin and the possibility of bacterial contamination and overgrowth over the vulva and vagina, some gynecologic laser surgeons advocate oral or topical antibiotic prophylaxis. This practice remains controversial, due to the lack of results of controlled studies, and has not been used by this author. Antibiotics have not been used for laser procedures on the cervix. The one exception to this is the patient who is found to have Bacterial Vaginosis at the preoperative evaluation. Because of the high bacterial count of anaerobic organisms in the vagina, this condition should always be treated with metronidazole or clindamycin before surgery.
Description of the Technique
Laser in the Treatment of Cervical Disease
When laser was first introduced as a tool for the gynecologist, it was the CO2 laser which was used for treatment of cervical disease.7 In the early reports of laser surgery, Baggish and Dorsey helped to define and establish the techniques used in CO2 laser therapy. They described using a 0.5–1 mm spot size and power of 25–50 W, resulting in a power density of 2,500–20,000 W/cm2 to cut, versus a 2–3 mm spot size and 20–25 W for vaporization which has a corresponding power density of approximately 500–800 W/cm2. This was done under colposcopic guidance with the laser coupled via a micromanipulator. In 1982, they reported a series of over 400 cases with CIN treated by laser with an overall cure rate of almost 96% at about 1-year follow-up.37 The only significant changes since then have been the use of slightly higher power densities. However moving above the 1,500 W/cm2 range frequently results in the beam cutting into vessels without first coagulating them and may cause significant bleeding. The higher power density does result in less thermal damage to the specimen, and offers a superior specimen for pathologic evaluation. There are very few current publications on this subject. Cochrane Systematic Reviews published on surgery for CIN and compared seven surgical techniques. They concluded that the Loop Electrosurgical Excision Procedure (LEEP) appeared to provide the more reliable specimen for pathology but the overall morbidity was lower with the laser conization. The limited evidence suggests that there is no obviously superior surgical technique for CIN.38
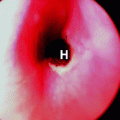
The KTP-532 laser has also been used for excisional and ablative procedures of the cervix, although there is little published data. The fiber is passed through a 9 in. hand piece with a 30° curve at the tip. This allows a free hand excision of the surgical specimen using 10–15 W (power density of 3,600–5,000 W/cm2). The most significant benefit is the marked decrease in bleeding encountered, which is generally the most difficult complication to deal with when using other modalities, including the carbon dioxide laser. This is explained by the high photochemical absorption of the 532 nm wavelength in the hemoglobin molecule. The beam passes through the relatively clear vessel wall, coagulating the blood before cutting it. Because of the forward penetration of this wavelength, the fiber is angled toward the patient and away from the specimen to decrease coagulation artifact and increase hemostasis during the incision of tissue. This author has used the KTP-532 preferentially for the past 15 years and found it allows for almost bloodless excisional cone biopsies1 see Fig. 1.
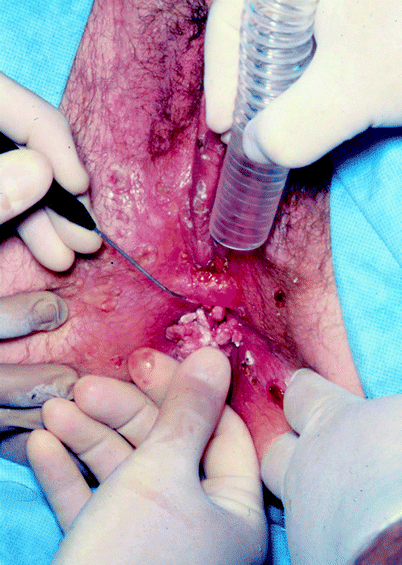

Fig. 1
The flexible quartz fiber of the KTP-532 laser is seen passing through a hand piece which allows a 30° angle at the tip. This allows the surgeon to apply the beam parallel to the skin surface for vulvar and vaginal procedures and is advantageous when performing excisional cone biopsies of the cervix
The Nd:YAG laser has also been used for excisional procedures, but because it must be coupled with a sapphire tip to do incisional work, it is somewhat more costly and difficult to manipulate inside the confined space of the vagina. It is, however, extremely hemostatic because of this wavelength’s intrinsic coagulation of protein.
Some authors suggest that combining LEEP with additional laser treatment of the cut margins and wound bed may improve long term success.39,40 Microscopically guided laser vaporization or laser excisional cone may be a less aggressive, and certainly more controllable, treatment modality than a traditional “cold knife cone (CKC)” and therefore, may be the choice for young women interested in preservation of fertility.41–43
In addition to the previously mentioned complications of CKC, it may result in the removal of too much or all of the endocervical glands resulting in cervical factor infertility and/or cervical stenosis, which precludes the passage of menstrual tissue. In the case of cervical stenosis, the CO2 laser is the instrument of choice to vaporize the scar tissue which is occluding the canal. A higher power density is used in the range of 5,000 W/cm2 to decrease thermal damage of the surrounding area, and allow the normal tissues to grow back into place.
The use of lasers has significantly decreased the complications, which have been historically encountered with the traditional cold knife cone.
Laser in the Treatment of Vaginal Disease
The treatment of vaginal dysplasia and condyloma remain challenging, regardless of treatment modalities. Since the vaginal epithelium is less than a millimeter thick, ablation has to be very superficial. Traditionally, the procedure was done in similar fashion to cervical laser vaporization, under colposcopic supervision with the micromanipulator. A 2 mm spot size is chosen at a 20–30 W power setting.6,44 Duane Townsend was among those establishing the technique. Because of the sharp tangential angulation of the impact beam delivered to the vaginal wall from a colposcopic delivery system, many surgeons today prefer to use a hand held device. This is incrementally better, but because of the bulky nature of the CO2 hand piece, it is still difficult to deliver a beam at 90° to the surface. The use of a fiber-optic laser such as the KTP-532 delivered through a hand piece with an angled tip as previously described, can allow the surgeon to deliver the beam with a more even distribution of energy to the surgical site. This is done using 10 W and short exposure times to compensate for the increased penetration of this wave length into the underlying tissue. With either technique it is very important not to overlap exposure applications in order to protect the underlying dermis.
The bulk of the available literature is from the 1980s to the mid-1990s. Uniformly, recurrence or persistence rates in the 30–50% range are reported after the first laser treatment. Even after the second and third treatment courses, 20% relapse or persist. This disease is considered multifocal in the majority of cases. In the case of VAIN III involving the vaginal cuff angles after hysterectomy, upper vaginectomy is proposed over plain laser ablation. Additional superficial lasing of the surrounding vagina is generally recommended.45–52 The main advantage of the laser procedure in comparison to conventional surgery or the use of cytotoxic agents, is the preservation of the anatomic integrity of the vagina, even after repeated laser courses.49 The recommended curative and safe depth to be achieved with the laser treatment is only 1.5 mm, which allows for reepithelialization without scarring.53
In the case of larger exophytic condyloma, the lesions are vaporized down to a level consistent with the surrounding vaginal wall before the adjacent normal appearing epithelium is treated. Because of the high recurrence rates, it is assumed that substantial areas of this normal appearing epithelium are in fact infected by HPV. In many cases of extensive disease, the correct plane of the original vaginal wall may be extremely difficult to determine. In this case, it is better to err in the direction of removing less tissue and, in some cases, to only treat part of the affected area. In many instances in which only partial vaporization is completed, the post-operative inspection reveals complete clearance of the condyloma. It is believed that these patients’ immune systems are stimulated to recognize the HPV as a result of the surgery and develop an immune response to the virus resulting in clearing of the untreated lesions.
Laser in the Treatment of Vulvar Disease
CO2 laser treatment for vulvar lesions was introduced about the same time as that of the cervix. The first reports were again by Baggish and Dorsey.54,55 They established the technique still in use, which employs a spot size of 2–3 mm and power settings of 15–30 W.
The goal is to confine the damage to the epidermis and upper papillary dermis; however, stacking of laser pulses by treating an area with multiple passes in rapid succession or by using a high overlap setting on a scanning device can lead to excessive thermal injury with subsequent increased risk of scarring. This untoward effect can be avoided by moving the beam in a serpentine fashion or in ever increasing concentric circles while avoiding overlap. The depth of ablation correlates directly with the cumulative amount of time × wattage, or work measured in joules, applied to a specific location. Using a power density of 800–1,000 W/cm2 is generally considered ideal, and should result in instantaneous boiling of the water in the epithelium, causing a vapor pocket above the dermis with elevation of the superficial layer. A power density, which is too high, results in deep vaporization into the dermis, which should be avoided. However this affect is time sensitive and as the skill and speed of the surgeon increase, a somewhat higher power density may be employed. If the power density is too low, an ablative plateau is reached with less effective tissue ablation and accumulation of thermal injury. This effect is most likely caused by reduced tissue water content after initial desiccation, resulting in less selective absorption of energy. The avoidance of pulse stacking and incomplete removal of partially desiccated tissue is paramount to prevention of excessive thermal accumulation with any laser system. The objective of ablative laser treatment is to destroy tissue down to the papillary dermis. Limiting the depth of penetration decreases the risk for scarring and permanent pigmentary alteration. When choosing treatment parameters, the surgeon must consider factors such as the anatomic location and proximity to vital organs. To reduce the risk of excessive thermal injury, partially desiccated tissue should be removed manually with wet gauze after each laser pass to expose the underlying dermis.
This technique is very reliable when treating the non-hairy vulvar surfaces. In areas containing hair, the method must be altered in an attempt to treat intraepithelial neoplastic disease, which progresses toward the base of the follicle. In most cases this is best done by making a second pass over this tissue after the superficial epithelium has been removed. This characteristic of VIN is felt to be one of the primary causes of persistent disease in cases which have otherwise been adequately treated by laser, and is perhaps the main reason some gynecologists still advocate excision in these areas. When cosmesis is a priority, the laser is still preferable, and the patient must be informed that she must commit to close follow-up and the possibility that further treatment may be needed. Unfortunately, because of the high recurrence rates in all VIN cases, this is more or less true for all patients.
When treating condyloma, the same techniques are generally applicable, but must be modified for larger exophytic lesions. The smaller condylomata may be simply vaporized or excised, but only to the level of the skin with care being taken not to coagulate the deeper dermis. The surrounding normal appearing epithelium is then treated in the same fashion as described above to a distance of 1–2 cm from the original wart. If this is not done, recurrence rates are very high.
For the larger pedunculated lesions, several techniques may be employed to decrease bleeding, which may occur if the CO2 laser is being used. Although the carbon dioxide laser is generally regarded as a very hemostatic instrument, it does so by thermal coagulation of vessels as the tissue is vaporized at the impact site, unlike the KTP-532 and Nd:YAG wavelengths which actually pass through water and coagulate by direct absorption in hemoglobin and protein respectively. When larger vessels are encountered, the CO2 may cut into the wall before the more rapidly moving blood is coagulated, creating bleeding. Further attempts to seal the vessel are then hampered by the complete absorption of the energy at the surface of the emerging blood, which does not allow heat to penetrate to the vessel below. The blood must be cleaned away while pressure is applied to the vessel in order to tamponade the bleeding to allow further progress. Alternatively, pedicles may be coagulated circumferentially before attempting excision. This is more productive if the blood flow can be stopped by pressure at the base. In some cases, it is better to use a much lower power density in the range of 200–400 W/cm2 to essentially cook the tissue initially. The wattage may be decreased or the spot size increased to accomplish the change. This can however, result in thermal spread into the dermis. In some instances, ectrocautery or sutures may be needed.
The KTP-532 laser, although much less widely available, may also be used in the treatment of these diseases, and offers significant advantages once the technique is mastered. Because this wavelength is not absorbed in water, there is deeper penetration (1–2 mm) than seen with the CO2. In order to decrease damage to the dermis, a higher power density (2,000–5,000 W/cm2) is used with a shorter application time, resulting in a more immediate coagulation of the epidermis, and less thermal spread. The outcome is somewhat different, because the effect will be desiccation and coagulation, with little or no vaporization. The treated epidermis is then wiped away using a gauze pad. The incidence of bleeding is much lower because of the extremely high absorption of the 532-nm wavelength in hemoglobin, and the ability to move through vessel walls, which are mainly water, before cutting into them. Should bleeding be encountered, the hand piece is backed away a few centimeters which will rapidly increase the spot size secondary to the 15° divergence of the beam from the fiber tip, resulting in a much lower power density for coagulation purposes. See Fig. 2a and b.
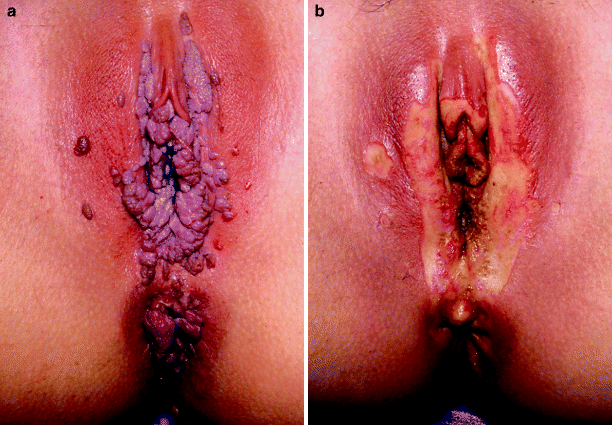

Fig. 2
(a) Patient with extensive condylomata acuminata of the vagina, vulva, and peri-anal skin. (b) Same patient following treatment using the KTP-532 laser. There is marked edema forming in the clitoral hood and labia minora before the entire procedure is completed. Note the absence of pitting into the dermis and the extended treatment area around all previously visible lesions
The most significant advantage to this wavelength is a marked decrease in postoperative pain compared to the CO2 laser. Significant and prolonged pain is the most common morbidity of vulvar laser treatment. Because the CO2 has very little forward scatter, the equivalent of a second degree burn is being created, with the intact sensory nerves exposed. Although the exact mechanism is uncertain, it is postulated that the 532 nm wavelength is absorbed in these nerve endings, essentially decreasing their response to stimuli for a short time during the healing process. There does not appear to be any long term effect. The increased depth of penetration may also be beneficial in treating VIN in skin containing hair follicles, although this has not yet been substantiated.
Laser therapy of vulvar intraepithelial neoplasia and condyloma results in excellent cosmetic and functional healing, but carries the risk of recurrent and persistent disease. Repeated treatments may be necessary. In light of the multifocal nature of these disease processes in the majority of patients, laser, with its ease of technique and low morbidity, is the treatment of choice, especially for younger women.5,44,56,57 For extensive VIN III, excisional laser surgery of the more suspicious areas combined with vaporization of the surrounding “normal” epithelium provides a histologic specimen and appears to be more effective than pure vaporization.57
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree