Glaucoma is a multifactorial optic neuropathy that is initially asymptomatic but results in progressive visual field deficits.
The prevalence of glaucoma increases with age, but can be seen at birth (i.e. congential). Intraocular pressure (IOP) elevation is a major primary risk factor.
The many types of glaucoma can be categorized into open-angle glaucoma (OAG) and closed-angle glaucoma (CAG).
Diagnosis of glaucoma requires measurement of IOP, assessment of vision loss by visual field testing, and examination of the ocular fundus for signs of optic neuropathy such as cupping, and differentiation of OAG and CAG by gonioscopy.
The only clinically proven treatment for glaucoma is lowering the IOP. This can be accomplished with medications, laser surgery, and/or incisional surgery.
Laser surgery has become increasingly popular as a treatment modality for glaucoma because the risks are favorable in comparison to incisional surgery. A number of lasers are used, the most common being argon, neodymium:yttrium-aluminum-garnet (Nd:YAG), and diode lasers. The specific laser used depends on the desired effect on the tissue.
Definition, Classification, and Epidemiology
The term “glaucoma” refers to a group of disorders that share common phenotypes. There are over 20 different subtypes of glaucoma. The glaucomas are defined by a characteristic loss of retinal ganglion cell axons leading to a progressive optic neuropathy that is related to intraocular pressure (IOP). If untreated, glaucoma can cause visual disability and even blindness. Although elevated intraocular pressure (IOP) is no longer formally part of the definition, it is recognized as the major risk factor for progression of the disease.
The subtypes of glaucoma are categorized into open-angle glaucoma (OAG) or closed-angle glaucoma (CAG). The “angle” refers to the iridocorneal (iris-cornea) junction at the periphery of the anterior chamber (Fig. 1). The angle is the site of drainage for aqueous humor. OAGs and CAGs are further subclassified into ‘primary,’ when the cause of the dysfunctional IOP is unknown, or ‘secondary,’ when the cause of the elevated IOP is the result of a known disease process. Furthermore, glaucomas are classified by their onset – acute or chronic. The most common form of glaucoma in the United States is Primary Open-Angle Glaucoma (POAG).
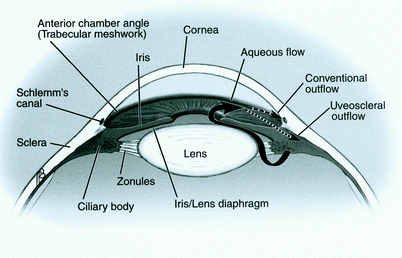

Fig. 1
Anterior segment anatomy and physiology
With approximately three million Americans affected by glaucoma it is the second leading cause of blindness in the United States. Although it affects people of all ages, it is 6 times more common in those over 60 years of age than those 40 years of age. Annual medical costs for glaucoma services to glaucomatous patients and glaucoma suspects totals over 2.86 billion dollars.
Aqueous Physiology and Pathophysiology
Aqueous humor is a clear fluid that circulates in the anterior chamber of the eye to provide nutrients and remove metabolic waste from the avascular structures of the eye – namely the lens, cornea, and trabecular meshwork. The balance of aqueous secretion and drainage determines the IOP. Aqueous humor is produced by the ciliary processes, which are located behind the iris, through active secretion, ultrafiltration, and diffusion. Aqueous circulates within the posterior chamber, travels through the pupil, and exits the eye through the angle via one of two pathways (Fig. 1): (1) the conventional pathway through the trabecular meshwork, canal of Schlemm, intrascleral channels, and then episcleral and conjunctival veins; or (2) the uveoscleral pathway, through the ciliary body face, choroidal vasculature, and vortex or scleral veins. The conventional pathway is responsible for the majority of outflow, especially in older adults. CAG results from physical obstruction of these drainage tissues by approximation of the iris and cornea (Fig. 2). OAG occurs when aqueous drainage is impaired by increased resistance to aqueous drainage that is intrinsic to the outflow pathways (Fig. 3). Although it is possible that overproduction of aqueous humor could lead to an elevated IOP, all studies have shown that the pathophysiology is poor aqueous drainage. The average IOP is approximately 16 mmHg (2 mmHg standard deviation). An elevated IOP is defined as a value that is 2SD above the average (i.e., greater than 20 mmHg). There is a form of OAG, named “low-” or “normal-tension glaucoma,” in which damage occurs within the average range (11–21 mmHg). Although IOP reduction is often effective treatment for this type of glaucoma, other etiologic factors such as vasospasm or ischemia are thought to have a larger role in the pathophysiology.
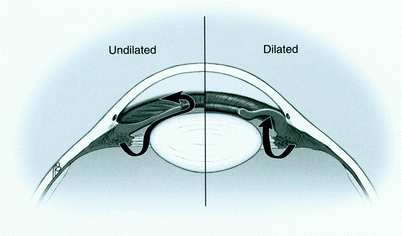
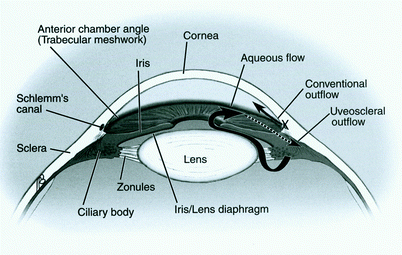

Fig. 2
Close-angle glaucoma due to pupillary block

Fig. 3
Open-angle glaucoma
Symptoms
Vision loss from chronic glaucoma is usually painless and slowly progressive. Peripheral vision is usually affected first, and the deficits may be asymmetric. This results in delays in realization of vision loss.
Acute angle closure and a few secondary glaucomas present with symptoms, most commonly painful red eye, blurred central vision, and rapid progression of visual loss. The presence of non-visually related symptoms are due to the rapid change in IOP causing immediate ischemic compromise of several ocular tissues – principally, the cornea and optic nerve.
Diagnosis
Diagnosis of glaucoma requires a complete history and ocular examination including measurement of IOP, assessment of the anterior chamber angle by gonioscopy, quantification of vision loss by visual field testing, and examination of the ocular fundus for signs of optic neuropathy such as cupping (Fig. 4). Gonioscopy is examination of the iridocorneal angle with a slit-lamp and contact lens containing mirrors to visualize the angle. Measurement of an elevated IOP identifies a significant risk-factor but is neither necessary nor sufficient for the diagnosis of glaucoma. Visual field defects and optic nerve defects characteristic of glaucoma are strong support for the diagnosis but other causes of optic neuropathy such as optic neuritis need to be excluded.
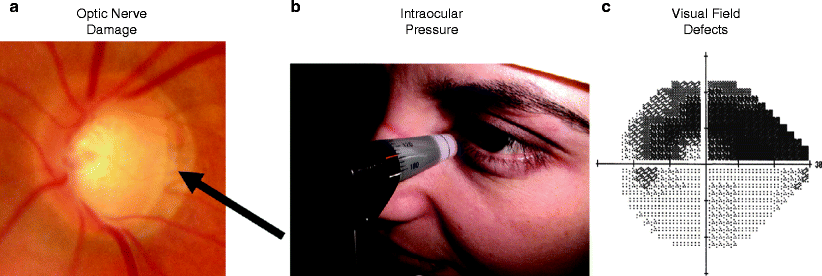

Fig. 4
Clinical triad of glaucoma, (a) optic nerve damage, (b) intraocular pressure, (c) visual field defects
Treatment
To date, lowering IOP is the only clinically proven treatment for the glaucomas. Glaucoma suspects may also be treated depending on the presence of high risk characteristics and the individual risk aversion of the patient. The treatment approach differs between CAG and OAG. CAG treatment requires laser or surgery to bypass the mechanical blockage. OAG can be treated with topical medications, laser, and/or incisional surgery. Topical medication may decrease aqueous production or increase aqueous drainage. Laser trabeculoplasty attempts to enhance the drainage function of the trabecular meshwork. Laser peripheral iridotomy creates a secondary pathway to allow aqueous to bypass a potential blockage; in doing so, equalization of the pressure gradient between the spaces anterior and posterior to the iris often allows the angle to deepen. Laser iridoplasty directly alters the angle anatomy by moving the iris away from the drainage structures. Glaucoma that is refractory to the above treatments may require cyclodestructive procedures to destroy the ciliary body and decrease aqueous production. Incisional operations such as trabeculectomy and glaucoma drainage implant devices create a new pathway to drain aqueous from the anterior chamber to the subconjunctival space.
General Comments Regarding Lasers in Glaucoma
Many lasers are used in glaucoma management. Their use has increased because their less invasive nature and generally lower rates of complications appeal to surgeons. The most commonly used lasers are argon, diode, and neodymium:yttrium-aluminum-garnet (Nd:YAG). The argon laser (488–514 nm) has a thermal effect on tissues, which either results in coagulation or vaporization depending upon the power settings used. The diode laser (810 nm) also has a photocoagulative effect. The Nd:YAG laser (1,064 nm) has a coagulative effect when used in a continuous-wave mode. The short-pulsed q-switched Nd:YAG has a photodisruptive effect on tissues, which has an explosive effect. Other lasers have a photoablative effect that results in excision of tissue without any damage to the adjacent tissue. Photoablation has more applications for the cornea, but is also used in glaucoma. Besides the type of effect observed on tissues, different lasers may be used because they specifically target a certain type of tissue or because they have a desirable depth of penetration.
Laser Iridotomy
Iridotomy is the creation of a microscopic hole through the iris that provides an alternate route for aqueous to enter the anterior chamber (Fig. 5). Laser iridotomy is preferred over surgical iridotomy because it is safer, equally effective, and preferred by patients; however, surgical iridectomy serves as second-line treatment if laser iridotomy is unable to be performed (e.g. a patient who is unable to maintain position in the laser). The popularity of this established technological advancement is evidenced by utilization statistics. Although the total number of laser iridotomies and surgical iridectomies has increased in proportion to the aging population, the ratio of laser iridotomies to surgical iridectomies performed has increased from 15:1 in 1995 to 52:1 in 2004. The procedure is treatment for all forms of CAG that involve pupillary block. Patients with easily occludable angles may also require the procedure.
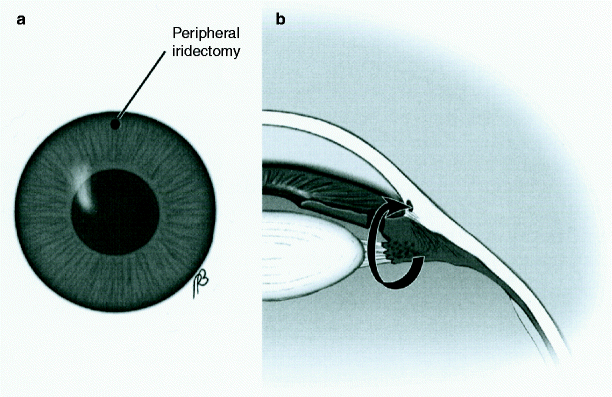

Fig. 5
Laser peripheral iridotomy, (a) shows clinical appearance of an iridotomy at the 12 o’clock position of the iris; (b) shows a secondary pathway to allow aqueous to bypass a potential blockage
Indications and Contraindications
Indications Closed-angle glaucoma with pupillary block Narrow angles with signs of glaucoma Narrow angles with positive provocative tests Contraindications Opacified cornea Uncooperative patient who is unable to maintain position for the procedure |
The primary indication for laser iridotomy is to relieve pupillary block that may progress to acute angle closure glaucoma or CAG. Mechanistically, pupillary block is caused by an increased resistance to aqueous flow through the pupil because of anatomic obstruction of the pupil by the lens or another anterior or posterior structure. Increased resistance leads to a pressure differential between the anterior and posterior chambers, which results in anterior bowing of the peripheral iris over the trabecular meshwork. Laser iridotomy is indicated if pupillary block has caused angle-closure or is in imminent threat of causing angle-closure. Angle-closure glaucoma may be acute, intermittent, or chronic; and all are indications for laser iridotomy. If narrow angles are identified, then the risks and benefits of treatment should be considered. For example, treatment would be indicated if there are signs of previous attacks or if the fellow eye has CAG. Additionally, patients with narrow angles can undergo tests to provoke angle-closure such as administration of a mydriatic agent, exposure to dark, or placement in the prone position. These tests may cause IOP elevation, and therefore may serve as an indication for treatment. Finally, in eyes where the clinician feels the angle is potentially occludable, laser iridotomy is indicated.
Specific causes of pupillary block include phacomorphic glaucoma (glaucoma caused by an excessively large lens), a dislocated lens, anterior protrusion of the vitreous face, occlusion by an artificial (pseudophakic) lens in the anterior chamber, posterior synechiae (adhesions of the central iris to the lens usually as a result of inflammation), or extreme miosis. This is in contrast to CAG without pupillary block such as vascular or inflammatory diseases that may cause peripheral anterior synechiae (adhesions of the peripheral iris to the cornea). However, patients with CAG without pupillary block may also be treated with laser iridotomy because some degree of pupillary block may be secondarily involved. Nanophthalmic (small eye) eyes frequently develop CAG because they have very small eyes relative to the size of their natural crystalline lens. Pupillary block related to an enlarged lens may be a contributing factor in these cases. The same reasoning may extend to patients with primarily an OAG. If a pupillary block component is suspected, the benefits of eliminating such a factor may outweigh the risks.
Contraindications to laser iridotomy are few and primarily include findings that increase the risk of complications from the procedure. Corneal burns may result from either (1) use of laser through an opacified cornea, or (2) use of laser in an eye with an extremely narrow angle. Acute CAG with pupillary block is ultimately treated with laser iridotomy; however, the procedure should ideally be done following the acute phase after the eye’s inflammation has had a chance to subside and the cornea has cleared. However, this is not always possible and laser iridotomy is still indicated if the cornea is clear enough to perform the procedure. Topical and systemic anti-glaucoma medications can acutely lower the pressure.
Techniques
Topical anesthesic and miotic medications are applied preoperatively. |
The argon, diode, or Nd:YAG laser is used to apply laser to the superior peripheral iris through a focusing iridotomy lens. |
The photocoagulative effect of argon laser is dependent upon pigmentation; therefore, techniques vary for irises of different colors. |
The Nd:YAG laser is photodisruptive and therefore does not dependent upon tissue pigmentation. |
IOP-lowering medications are used perioperatively. Corticosteroids may be temporarily used postoperatively to control inflammation. |
Pre-operative Management
Topical proparacaine is sufficient to provide anesthesia. A miotic agent is applied topically to thin the iris and pull it away from the angle. This allows for easier penetration and minimizes corneal endothelial injury. An Abraham iridotomy lens will help stabilize the eye, keep the eyelids open, provide a magnified view, and minimize corneal burns by acting as a heat sink and increasing the power density of the laser at the iris. The iridotomy site should be made in a relatively thin region of the iris. Typically, the iridotomy site is between the 11 o’clock and 1 o’clock positions where the lid is likely to cover the iridotomy site and therefore reduce the risk of visual disturbances (e.g. glare or shadow image) from light entering through the iridotomy site. However, others have successfully used the temporal or nasal regions of the iris without noticeable issues with visual disturbances.
Description of the Technique
Q-switched Nd:YAG laser, the argon laser, and diode lasers can be effectively used for iridotomy. This review will focus on q-switched Nd:YAG and the argon laser because these two are most commonly used in practice. Each has unique properties that affect the selection of laser type and use of the laser for different colored irises. The q-switched Nd:YAG creates the iridotomy by photodisruption, an optical breakdown of molecules into their component ions resulting in explosive disruption and essentially excision of tissue. One advantage of photodisruption is that it does not depend upon tissue absorptivity and therefore is equally effective for different colored irises. One disadvantage of photodisruption is its lack of coagulative effect. On the other hand, argon laser has a thermal effect and therefore results in photocoagulation or photovaporization, the specific effect depending upon the duration of exposure and energy density of the laser used. The thermal effect is beneficial in that it can provide coagulation. The disadvantage of argon laser is its dependence upon absorption by tissue pigments. Argon laser is ideal for medium brown irises, but may have a charring effect on dark brown irises and poor absorption in blue irises. As a result of the above differences, q-switched Nd:YAG laser is simpler to use, and argon laser iridotomy techniques vary for irises of different color. Specific descriptions of the techniques follow.
Q-switched Nd:YAG lasers have a wavelength of approximately 1,064 nm and can be used at a range of power densities depending upon the number of bursts. Typically, there are one to three pulses per burst with each burst delivering 1–10 mJ. The focal point of the laser should be within the iris stroma to avoid corneal damage from the explosive effect. The iridotomy site should be at least 0.1 mm.
The effects of argon laser vary for tissues with different levels of pigmentation, therefore different techniques have been employed. The darker the iris color (i.e. greater amount of melanin in the stroma), the greater the absorption of the laser energy. Thus, the darker colored iris will require less energy to achieve the same results. Typical settings range between 600 and 1,000 mW with a spot size of 50 μm with a duration of 0.02–0.05 s. The pit that is initially formed can be enlarged to a diameter of 0.2 mm with 30–70 pulses. Light blue irises have little pigment anteriorly (in the stroma) but the same iris pigment epithelium as brown irises posteriorly. As a result, the argon laser may penetrate the iris pigment epithelium but leave the stroma intact. A variety of creative techniques can be used to avoid this. One approach is to use longer exposures that generate heat that transmits to the stroma and creates a bubble. Laser entering through the apex of the bubble will repeatedly reflect within the bubble and more effectively ablate the stroma.
Although the first laser iridotomies were performed using the argon laser alone, in the modern era, either the Nd:YAG laser will be used alone or a combination an argon laser. Use of the two lasers minimizes the risk of hyphema and total energy delivered. Argon laser is used to for photocoagulation to increase tissue density and minimize the risk of bleeding. The Nd:YAG laser is then used for photodisruption.
Post-operative Management
Intraocular pressure is generally checked 30–120 min after the procedure. Apraclonidine or brimonidine are given perioperatively to mitigate elevations in pressure. Topical steroids may be given for several days post-operatively to control inflammation. If the angle remains narrow, laser peripheral iridoplasty may be considered, which will be described in the next section.
Adverse Events
Adverse events | Management |
Iritis | Corticosteroids if more than mild |
Increased intraocular pressure | Topical medications (α-adrenergic agonists, β-blockers, osmotic agents, or carbonic anhydrase inhibitors) |
Cataract | No treatment |
Hyphema | Pressure with contact lens for homeostasis |
Corneal damage | No treatment |
Failure to perforate | Retreatment after pigment cloud has dispersed |
Late closure | Retreatment |
Retinal burn | Avoid with standard precautions |
Most side effects of laser iridotomy are often minimal and self-limited. Iritis occurs and is treated by post-operative corticosteroids or topical non-steroidal anti-inflammatory treatment. Persistent iritis may be related to a preexisting uveitis. Intraocular pressure elevations are common 1–2 h after the procedure but are usually self-limited and resolve within 24 h. Topical medications to lower IOP can be used to limit IOP elevation. Filtering surgery may be required if more severe and sustained elevations occur, more commonly in eyes with a component of OAG.
Most complications can be avoided with appropriate precautions and careful technique. Cataract may occur and is more easily formed by the q-switched Nd:YAG laser if it is applied to an open iridotomy site because the effect of the laser does not depend upon pigmentation. Hyphema is also much more common with q-switched Nd:YAG laser because it does not have a coagulative effect. Applying pressure with the contact lens usually provides sufficient homeostasis. Corneal damage may occur from either argon or Nd:YAG laser iridotomy. If the iridocorneal angle is closed or extremely narrow, the endothelium may be affected. No treatment is generally required, but if the iridotomy is incomplete, a new site may be selected. Closure of the iridotomy site may occur if the site is small or if there is an underlying uveitis.
Argon Laser Peripheral Iridoplasty
Argon laser peripheral iridoplasty is the delivery of thermal energy that causes circumferential contraction of the iris away from the trabecular meshwork. Laser iridoplasty is a treatment for certain closed-angle glaucomas, and is often attempted when laser iridotomy fails or is not indicated because the pathophysiology does not involve pupillary block.
Indications and Contraindications
Indications Closed-angle glaucoma without pupillary block (e.g. plateau iris syndrome) Preceding laser iridotomy for CAG with pupillary block and inflammation Preceding laser trabeculoplasty for focal areas of angle narrowing Contraindications Severe corneal edema Peripheral anterior synechiae Corneal opacities – treatment through the opacity is not recommended |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree