Fig. 1
Absorption curves of melanin, oxyhemoglobin and water (From Bolognia et al. Dermatology. 2003)100
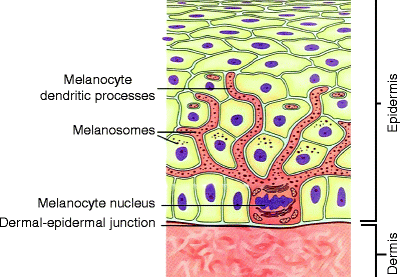
Fig. 2
Melanocyte residing within the epidermis. Melanosomes extend throughout the dendritic processes and are transferred to neighboring keratinocytes (Modified from www.freethought-forum.com)
While electron microscopy has confirmed highly selective destruction of melanosomes within melanocytes and melanized keratinocytes, it is not know precisely how the pigment-containing cells are destroyed. It is believed that destruction of melanocytes and melanized keratinocytes are destroyed due to mechanical damage from acoustic waves that emanate from the absorbing melanosome.8,9 Damage to these cells results in vacuolization and deposition of pigment and nuclear material at the cellular periphery. In addition, subepidermal vesiculation may occur at the level of the lamina lucida. Following damage, repigmentation results from migration of residual melanocytes from either adnexal structures or adjacent unirradiated skin.
When treating pigmented lesions, Q-switched lasers generate an immediate ash-white color at the site of impact. The cause of this tissue response is due to heat induced steam cavities in melanosomes which cause a scattering of visible light, producing a white color.10 Well demarcated, circular, highly reflective structures of 1–30 μm within melanosomes have been identified by confocal microscopy, electron microscopy, and optical coherence tomography (Fig. 3). These circular structures are presumed to be gas bubbles and gradually disappear over 20 min, correlating to the disappearance of the clinical ash-white color over the same period of time.12 The exact content of the gas bubbles is not know, but may be water vapor, nitrogen, or other gases. The adequate laser exposure dose for melanosome damage correlates well with the clinical threshold for immediate skin whitening. In other words, if the clinical ash-white color is not visible, the laser exposure dose is not sufficient. Darker skin has a lower threshold for whitening due to a higher epidermal melanin content.12 With increasing wavelengths, melanin absorption decreases, and the required threshold laser exposure dose increases.13,14 Subthreshold fluences appear to actually stimulate melanogenesis because of activation of epidermal melanocytes after non-lethal injury (Fig. 4).15
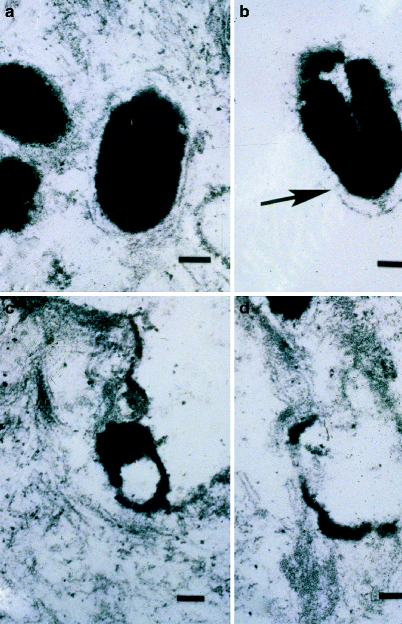
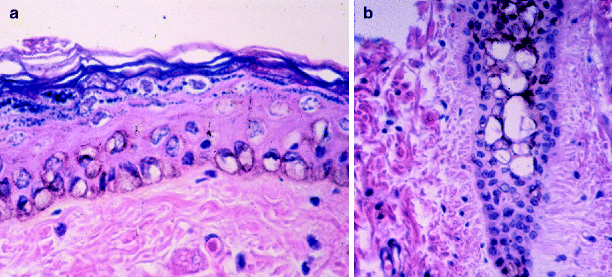

Fig. 3
Electron micrograph obtained immediately after Q-switched ruby laser irradiation. The targeted melanosome shows membrane disruption with disorganization of its internal contents (a) prior to irradiation; (b) immediately after irradiation showing early melanosome disruption; (c) more disruption; (d) almost complete disruption of a melanosome immediately after irradiation (From Ardnt et al.11)

Fig. 4
Histologic appearance of black guinea pig skin immediately after irradiation with the Q-switched ruby laser. (a) Characteristic “ring cell” formation in the basal lamina, representing melanocytes and keratinocytes with condensed nuclear and pigment material at their peripheries. (b) Similar changes in a hair follicle (From Ardnt et al.11)
Q-Switched and Pulsed Lasers and Light Sources
Selective damage to melanosomes in human skin was first demonstrated with a 351 nm XeF excimer laser delivering 20 ns pulses.16 Although light at 351 nm is well absorbed by melanin, this short wavelength only penetrates less than 100 μm into the skin due to light scattering.17 It was subsequently found that selective melanosome damage could be produced by also the pulsed tunable dye laser13,18 (wavelength 435–750 nm, pulse width 300–750 ns), Q-switched ruby laser6 (wavelength 694 nm, pulse width 40 ns) and the Q-switched neodymium:YAG laser14 (wavelength 355, 532, and 1,064 nm, pulse width 10–12 ns). While shorter wavelengths, such as 351 nm are better at absorbing melanin, longer wavelengths penetrate deeper into the skin, increasing their ability to reach deeper melanosomes (Fig. 5).
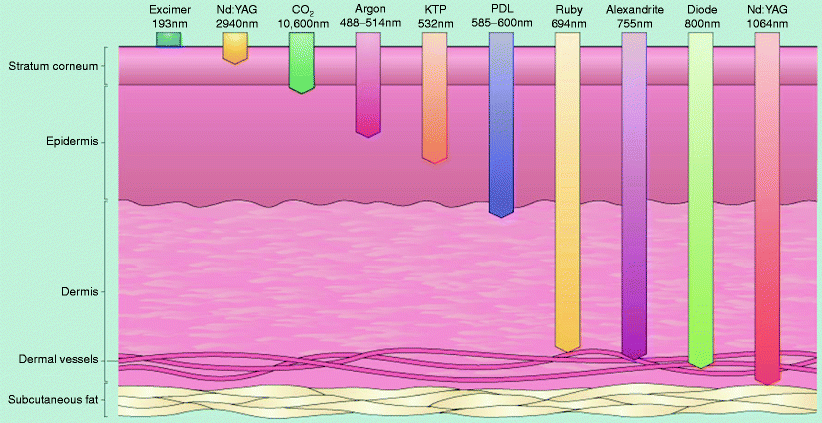

Fig. 5
Laser depth of penetration. Depth of penetration for lasers of varying wavelengths. For lasers in the visible and near infrared ranges, the depth of penetration increases as the wavelength increases (From Bolognia, et al. Dermatology. 2003)
Several lasers can be used today to treat pigmented skin lesions (Table 1). These include lasers that are: (1) pigment nonselective, (2) highly pigment selective, (3) and those that are somewhat pigment selective.
Table 1
Lasers in the treatment of pigmented lesion
Device | Manufacturer | Laser type | Wavelength (nm) | Pulse duration | Spot size (mm) | Maximum repetition rate(Hz) | Comments |
|---|---|---|---|---|---|---|---|
Spectrum RD-1200 | Palomar | QS Ruby | 694 | 28 ns | 5, 6.5 | 0.8 | Large spot size promotes deeper penetration |
EpiTouch | Lumenis | QS Ruby | 694 | 25 ns | 5 | 0.8 | Long pulse mode available for hair removal |
Sinon | Wavelight | QS Ruby | 694 | 15–40 ns | 3–9 | 20 | Long pulse mode available for hair removal |
AlexLAZR | Candela | QS alexandrite | 755 | 50 ns | 2, 3, 4 | 5 | Fiberoptic delivery system |
VersaPulse VPC | Lumenis | FD Nd:YAG | 532 | 2–50 ms | 2–10 | 6 | Four lasers within one box |
QS FD Nd:YAG | 532 | 4 ns | 2–6 | 10 | |||
QS Nd: YAG | 1,064 | 5 ns | 2–6 | 10 | |||
QS alexandrite | 755 | 45 ns | 2–6 | 10 | |||
Medlite C6 | HOYA | QS FD Nd:YAG | 532 | 5–20 ns | 2, 3, 4, 6 | 10 | Handpiece converts λ to 585 and 650 nm |
ConBio | QS Nd : YAG | 1,064 | 5–20 ns | 3, 4, 6, 8 | 10 | ||
Alex | Candela | QS FD Nd:YAG | 532 | 50 ns | 2, 3, 5 | 5 | |
TriVantage | QS alexandrite | 755 | 2, 3, 4 | 5 | |||
QS Nd:YAG | 1,064 | 2, 3, 5 | 5 | ||||
SkinClear | Sybaritic | QS FD Nd:YAG | 532 | 10 ns | 1, 2, 3 | ||
QS Nd:YAG | 1,064 | 10 ns | 1, 2, 3 | ||||
Naturalase | Focus | QS FD Nd:YAG | 532 | 10–20 ns | 7 | ||
Medical | QS Nd:YAG | 1,064 | 10–20 ns | 7 |
Pigment Nonselective Lasers
The carbon dioxide4,19 (10,600 nm), erbium-YAG (2,940 nm) and Erbium xxx (1,540 nm) and yttrium scandium gallium garnet (YSGG) (2,790 nm) lasers are pigment nonselective lasers that remove epidermal pigment because of their ability to target water and ablate the entire epidermis, including melanocytes and melanized keratinocytes. Earlier devices removed a relatively uniform layer of the epidermis and with the epidermis went the associated pigment. Newer fractionated laser technologies damage columns of the epidermis and dermis while leaving interspersed areas unaffected. In this manner, there is faster healing as the surrounding normal tissue heals each light column of damage (Fig. 6). The fractionated CO2 and fractionated erbium:YAG lasers work in the same manner as their non-fractionated counterparts but deliver the light in many small columns. Because only fractions of the pigmented epidermis are affected, it stands to reason that a series of treatments is necessary to achieve the desired result and at least some of the original pigmented epidermal lesion would remain even after a series of treatments resulting in incomplete lesion removal.
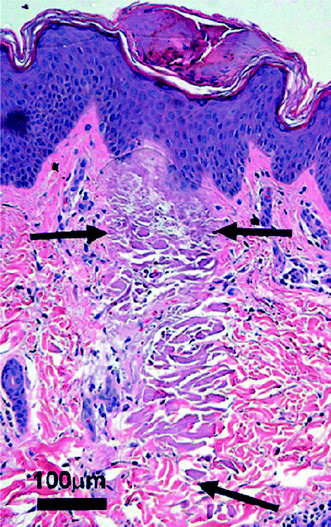

Fig. 6
Fractionated erbium glass laser causing tissue coagulation in a narrow microthermal zone of injury (From Vikramaditya et al.20)
The YSGG laser is a non fractional device that targets epidermal water and indirectly associated melanin. Plasma skin resurfacing is not a laser but is a device that utilizes radiofrequency energy to convert nitrogen gas into plasma. The plasma gives up energy to the skin and there is rapid heating of the skin into the dermis. Depending on the amount of energy delivered a thinner or thicker layer of the epidermis and adjacent dermis is affected by the treatment. Over 24–48 h the epidermis is shed, including melanocytes and pigment laden keratinocytes, and is then regenerated, leaving behind a new epidermis with less unwanted sun induced pigment.
Highly Pigment Selective Lasers
Older technologies, such as continuous wave (CW) and quasi-CW visible light lasers, including the argon laser (488, 514 nm), copper vapor laser (511 nm), and krypton laser (521, 530 nm), can be used to selectively remove epidermal pigmented lesions. However, spatial thermal injury confinement is not possible and unaffected adjacent skin may also be damaged. The risk/benefit ratio is higher with these CW and quasi-CW devices.
There are three short-pulsed, pigment selective lasers that are widely used today: (1) the Q-switched ruby laser (QSRL) (694 nm), (2) the Q-switched alexandrite laser (755 nm), and (3) the Q-switched neodymium:YAG (Nd:YAG and KTP) laser (1,064, 532 nm). These lasers selectively target melanin by delivering high-intensity, short-pulsed radiation at varying wavelengths. “Q-switched” is an abbreviation for “quality-switched” and refers to lasers which release an extremely high powered pulse (109 W) with an ultra-short pulse duration. Through the use of an optical shutter, these lasers store large amounts of energy in the laser cavity and then release the stored energy when the laser fires.
The QSRL emits red light at a wavelength of 694 nm and a pulse duration of 28–40 ns (Table 1). Light is delivered through a mirrored articulated arm at a spot size of 5 or 6.5 mm and a repetition rate of 2 Hz. The Q-switched alexandrite laser has a near infrared wavelength of 755 nm, pulse duration of 50–100 ns, spot size of 2–4 mm, and a repetition rate up to 10 Hz. Depending on the exact device, light is delivered either through an articulated arm or through a semi-flexible fiber optic cable. The Q-switched Nd:YAG laser emits infrared light at 1,064 nm. The wavelength can be halved by placing a frequency-doubling KTP (potassium-titanyl-phosphate) crystal in the laser beam’s path. Dye-impregnated handpieces can convert the 532 nm wavelength to either 585 nm (yellow) or 650 nm (red). An articulated arm delivers pulses with a spot size to 1.5–8 mm, a pulse duration of 5–10 ns, and a repetition rate up to 10 Hz.
Long-pulsed (millisecond rather than nanosecond domain) 532 nm (KTP) Nd:YAG lasers and 595 nm pulsed-dye lasers, which are traditionally used to treat vascular lesions, can also be used to treat superficial pigmented lesions. However, the long pulse width of these lasers is close to the thermal relaxation time of the entire epidermis (about 10 ms)21 and therefore does not allow for selective damage to melanosomes. Because of their longer pulse width, millisecond domain lasers produce a purely thermal effect on their target, unlike the photomechanical effect of Q-switched lasers. The target in this case may in fact be melanocytes rather than melanosomes. Regardless, these longer pulse duration devices, just like intense pulsed light devices are highly effective in removing unwanted epidermal pigment. These lasers are not suitable for treating dermal pigmented lesions because of the limited penetration depth.22 Although no longer manufactured, a pigmented lesion pulsed dye laser (non-Q-switched) used a xenon flashlamp to pump a coumarin-containing dye that expelled pulses of green light at 510 nm. Although this laser was useful for epidermal lesions, its shallow penetration made it much less effective on dermal lesions.
The Q-switched ruby, alexandrite and Nd:YAG lasers also have long-pulsed counterparts with the same wavelengths that operate in a normal (non-Q-switched) mode. These normal mode lasers are often used for laser hair removal because their higher fluences and longer pulse durations target large pigmented structures such as hair follicles or nests of cells rather than individual melanosomes or pigmented cells.23 Normal mode lasers have been shown to be effective in the removal of epidermal pigmented lesions but are not ideal because damage may be imparted on surrounding tissue.
Non-coherent light sources (intense pulsed light or IPL) can also be used for pigment removal. Polychromatic light is emitted ranging from 515 to 1,200 nm (visible to infrared) and filters are used to cut off the light above or below predetermined wavelengths. Since melanin exhibits a broad absorption spectrum, monochromatic laser devices are not necessary to target superficial melanin. The shorter wavelengths emitted by IPL devices are highly absorbed by melanin. IPL devices release light as a series of single, double, or triple pulses (millisecond domain). Like the millisecond domain lasers, the millisecond pulse width of IPL devices approximates the thermal relaxation time of the epidermis (10 ms) and produces a photothermal, not photomechanical effect, on its target. To avoid damage to normal surrounding epidermis, most IPL devices have skin cooling during treatment to protect the epidermis from excessive thermal injury. The IPL devices should not be used for dermal pigmented lesions.
Indications and Contraindications
Ephelides, lentigines, and café au lait macules, are common epidermal pigmented lesions that respond to laser and light treatment.
Melanocytic nevi, nevus of Ota and other dermal melanocytoses, melasma, post-inflammatory hyperpigmentation, and drug induced pigmentation are dermal pigmented lesions that can be treated with lasers. Of these, only nevus of Ota and other dermal melanocytoses respond predictably and favorably.
Shorter laser wavelengths may suffice for epidermal lesions as deep tissue penetration is not necessary.
Long wavelengths are required for dermal pigmented lesions.
Multiple treatments are often needed for complete removal.
Anesthesia may be needed for larger or dermal lesions.
Epidermal Pigmented Lesions
Many clinical studies have proven the efficacy and safety of Q-switched lasers24–26 and the 510 nm pulsed dye laser27 in the treatment of various epidermal pigmented lesions, including: ephelides, lentigines, Café au lait macules, seborrheic keratoses, nevi spilus, and Becker’s nevi. Since pigment in epidermal lesions is found superficially, shorter-wavelength devices can be used successfully despite their limited penetration depth. For example, the 510 nm wavelength of the pigmented lesion pulsed dye laser and 532 nm pulsed lasers are highly absorbed by melanin but penetrates only about 250 μm into the skin.17 The Q-switched ruby and alexandrite lasers effectively treat both epidermal and dermal pigmented lesions since their wavelengths are still within the melanin absorption spectrum and they penetrate deeply into the dermis. The Nd:YAG (1,064 nm) laser penetrates deeply but is poorly absorbed by melanin, making the 532 nm wavelength preferable for epidermal lesions. When using the 510 nm and 532 nm wavelengths, hemoglobin competes with melanin for absorption of light. Nanosecond pulses at these wavelengths causes rupture of superficial blood vessels, manifesting clinically as purpura.13
Lentigines
Lentigines are extremely common hyperpigmented macules that are most often due to chronic sun exposure and are then referred to as solar lentigines. On pathology lentigines display increased single melanocytes along the basal layer with elongation of club-shaped rete ridges. In addition to solar lentigines, there are lentigines associated with a syndrome (e.g., Peutz-Jeghers (Fig. 7)) and labial melanotic macules (labial lentigines). All three Q-switched lasers are highly effective for treating all types of lentigines.28,29 Both 35% trichloroacetic acid peels and cryotherapy are inferior to Q-switched lasers in the treatment of lentigines.30,31 With one treatment using a Q-switched laser, at least 50% clearing of lentigines is expected, and additional treatments may be utilized to remove remaining pigment (Figs. 8 and 9). Although less selective, non Q-switched (millisecond domain) KTP, 595 nm pulsed-dye, ruby, alexandrite, and diode lasers may also be used to treat lentigines. A study of Asian patients with lentigines found a long-pulsed KTP (532 nm) laser (without skin cooling) to be as effective as a 532 nm Q-switched Nd:YAG laser, and with less risk of post-inflammatory hyperpigmentation. Additionally, a study using a 595 nm pulsed-dye laser on lentigines in Asian patients showed a mean of 82% improvement in lentigines by reflectance spectrometry.22 Several studies have also demonstrated the effectiveness of broad band light sources (Intense Pulsed Light) in clearing lentigines. Adjunctive use of 5-aminolevulinic acid (5-ALA) with IPL provides greater improvement of epidermal pigment than with IPL alone.32–34
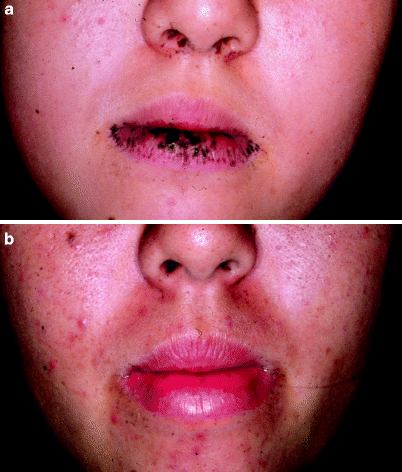
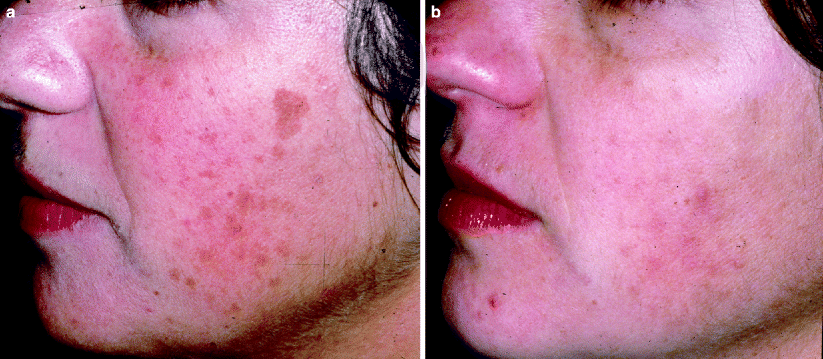
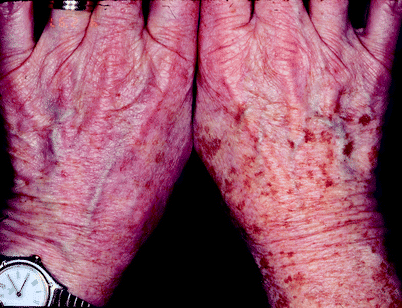

Fig. 7
(a) A patient with Peutz-Jeghers syndrome with extensive macular pigmented lesions over the lip prior to treatment. (b) After two treatments with the Q-switched ruby laser, the lesions are completely cleared (Courtesy Tadashi Tezuka, MD)

Fig. 8
(a) A woman in her early 40 s with significant photoaging and numerous lentigines prior to treatment. (b) Six weeks after one single treatment with a Q-switched ruby laser. There is about 70% improvement of the lesion. No further treatments were requested by the patient

Fig. 9
A patient’s right hand with copious solar lentigines and the patient’s left hand with significantly fewer solar lentigines after two Q-switched alexandrite treatments
Café Au Lait Macules
Café au lait macules (CALMs) are well circumscribed, homogenous light brown macules that occur as isolated lesions in the general population. Their prevalence varies amongst ethnicities but ranges from 0.2% to 18%.35 Café au lait macules may also be found as multiple lesions in association with a syndrome (e.g., neurofibromatosis, Noonan syndrome). Histologically, there is an increase in melanocytes along the basal layer, hypermelanosis of melanocytes and keratinocytes, and giant melanin granules. Treatment of CALMs with lasers is minimally successful and often unpredictable.36 Temporary lightening or clearing can be achieved after multiple treatments (Fig. 10). However, recurrences are seen in up to 50% of treated lesions, even when clearing is initially achieved. Post-inflammatory hyperpigmentation is frequent following laser treatment of CALMs, especially in patients with darker skin types. Alster demonstrated complete elimination of most CALMs after an average of 8.4 treatment sessions with the 510 nm pulsed dye laser, indicating that multiple treatments are needed for complete resolution.37,38 Er:YAG resurfacing has also been shown to eliminate CALMs.38
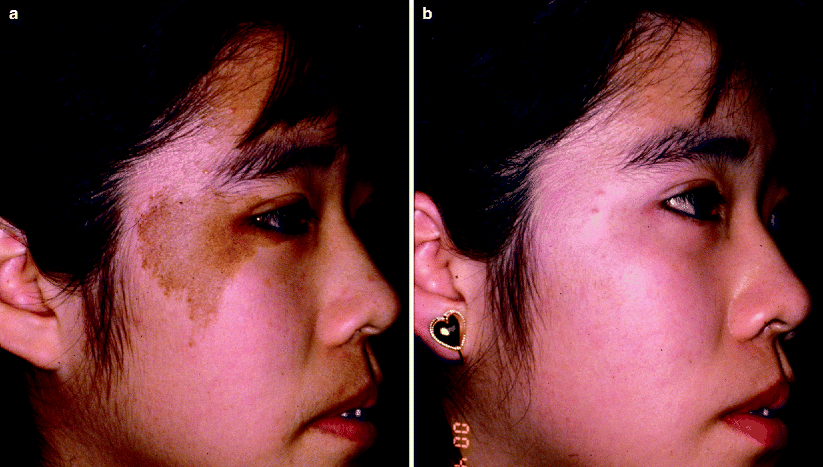
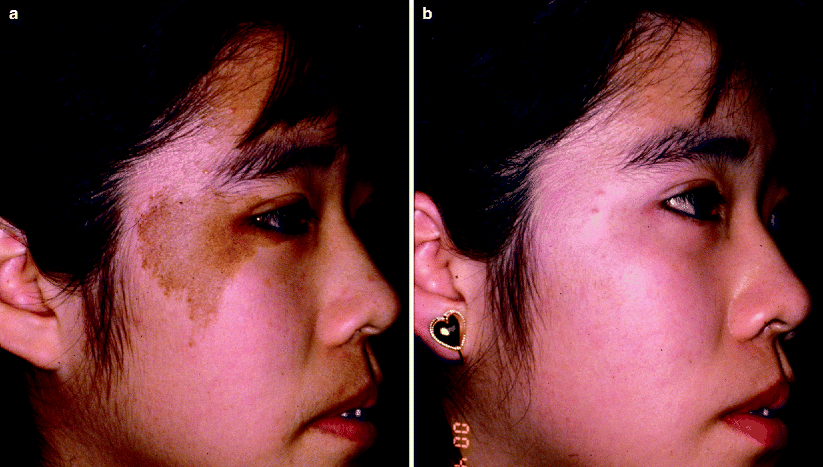
Fig. 10
(a) A young Asian patient with a café au lait macule on her right cheek. (b) After a series of treatments with the Q-switched laser, the café au lait macule has cleared markedly
Other Epidermal Lesions
Ephelides (freckles) are hyperpigmented small macules located on sun-exposed skin and become darker in the summer and lighter in the winter. There is no increase in the number of melanocytes on pathology, but there is an increase in melanin. Ephelides respond well to Q-switched laser treatment (Fig. 11). Another common lesion, seborrheic keratoses, may respond to laser treatment. In general, thinner seborrheic keratoses respond better to laser treatment than thick lesions.27 Cryotherapy or cryotherapy in combination with laser treatment is preferred to laser treatment alone for thick seborrheic keratoses. A nevus spilus (speckled lentiginous nevus) consists of a background CALM and scattered nests of nevi cells. Successful clearing of the darker nevocellular component has been reported with the QSRL, but the CALM component tends to recur.24 A Becker’s nevus is a hyperpigmented, hair-bearing plaque that most commonly occurs on the upper trunk or shoulder of males. These lesions may also be associated with a dermal smooth muscle hamartoma. The hyperpigmented component of Becker’s nevi respond similarly to laser treatment as CALMs, having frequent recurrences (within 6–12 months) and post-inflammatory hyperpigmentation.39
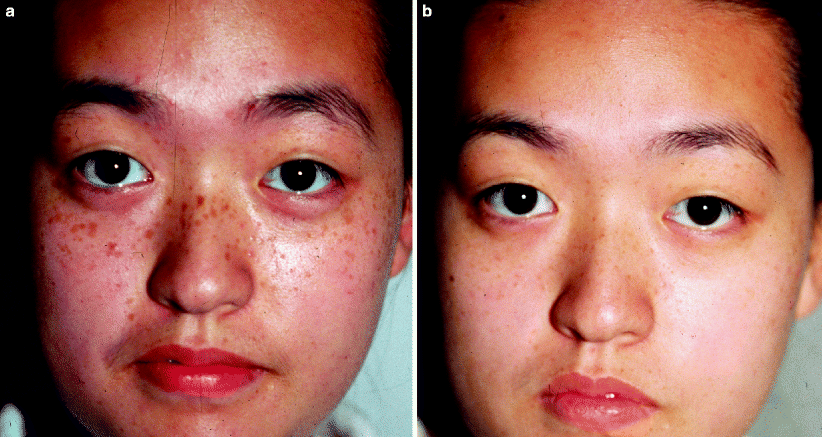

Fig. 11
(a) Japanese male with extensive numbers of freckles prior to treatment. (b) After treatment with a Q-switched ruby laser, the freckles are entirely clear (Courtesy of T.K. Lee, MD)
Dermal Pigmented Lesions
Q-switched lasers have revolutionized the treatment of dermal pigmented lesions including: melanocytic nevi, nevus of Ota, and melasma. Prior to the advent of Q-switched lasers, these lesions were treated with nonspecific destructive means such as excision,40,41 dermabrasion,42 salabrasion,43 cryotherapy,44 solid carbon dioxide ice,45 or continuous wave laser ablation.46 These older methods were ineffective and often caused scarring or dyspigmentation. By selectively targeting dermal melanin, Q-switched lasers provide effective treatment of these lesions without risking textural or pigment alteration. The Q-switched ruby, alexandrite and 1,064 nm Nd:YAG are the most commonly used lasers. All of these lasers are still within the absorption spectrum of melanin yet also have wavelengths that are long enough to penetrate into the dermis. Broad band light sources (such as IPL) lack wavelength specificity and have longer (ms range rather than nanosecond range) pulse durations, making them unsuitable for treating dermal pigmented lesions.
Melanocytic Nevi
Laser treatment of melanocytic nevi is controversial since it is unclear whether laser irradiation has any potential to induce malignant change in nevus cells. In vitro studies of melanoma cells treated with Q-switched lasers have found changes in cell surface integrin expression, with subsequent alteration of cell migration.47 Another in vitro study found a significant increase in p16INK4a in p16 positive cell lines following irradiation with Q-switched laser light and suggested that sub-lethal laser damage may increase DNA damage leading to an increase in p16 expression.48 In clinical practice, benign appearing nevi that tend to recur following laser treatment may show newfound clinical and histologic atypia, referred to as pseudomelanoma.49 Despite this, there has never been a report of true malignant transformation of a benign pigmented lesion following laser treatment.50 Theoretically, laser treatment of melanocytic lesions may decrease the risk of malignant transformation of a benign pigmented lesion simply by reducing the population of existing potentially premalignant cells. One study reported that no significant malignant markers (such as proliferation cell nuclear antigen, pyrimidine dimers, 8-OhdG, and p53) were found following treatment of nevi with Q-switched lasers.51 While these findings are notable, additional study is needed to assess the outcome of Q-switched laser treatment of nevi. Until more is known, it is prudent to perform a biopsy prior to laser treatment to confirm the benign nature of the nevus. Laser treatment should not be performed on nevi in patients with a personal or family history of malignant melanoma.
The Q-switched ruby, alexandrite, and 1,064 nm Nd:YAG lasers all have some efficacy in removing flat or slightly raised acquired nevi.52–54 Lighter nevi respond best to shorter wavelengths that maximize melanin absorption, while darker nevi typically respond to any wavelength within the melanin absorption spectrum. Multiple treatments are frequently necessary for optimal lightening. Clinical lightening is also associated with the development of a subtle microscopic scar up to 1 mm thick that obscures residual nevus cells. It is often unfeasible to attain complete resolution of nevi,54 and recurrence after laser treatment is common. There may be persistence of nevus cells containing little pigment located in the deeper dermis that are shielded from laser radiation by the more pigmented superficial cells.52 Q-switched laser radiation does not penetrate sufficiently to effectively treat thicker papillated or dome-shaped dermal nevi. The short pulsed erbium:YAG laser has been reported to be quite effective in removing flat or slightly palpable melanocytic nevi. Single pulses of 5.2–14 J/cm2 were found to clear 27 of 28 nevi on follow-up and histological examination.55 The QSRL has been reported to successfully eliminate flat blue nevi.56
Although Q-switched lasers may effectively lighten congenital nevi, there is frequently repigmentation due to persistence of nevus cells within the deeper reticular dermis and within adnexae.50,57 In a split nevus study on 15 patients, Kono et al. showed greater clearing with combined Q-switched and normal-mode ruby laser (NMRL) than in NMRL alone. They also showed a marked decrease in nevus nests at the dermal-epidermal junction, papillary and reticular dermis. In theory, millisecond-domain pulses are more appropriate than Q-switched pulses for treating thick lesions such as congenital nevi because they produce less selective thermal damage, destroying entire nests of cells rather than individual pigmented cells. Japanese investigators have reported impressive long-term clearing of congenital nevi treated with the millisecond-domain normal-mode ruby laser.23,58 However, other investigators have reported poor results treating congenital nevi with both Q-switched and normal mode ruby lasers.59 Long-pulse ruby lasers also offer the potential to reduce the amount of hair within congenital nevi. In Japanese studies, no histological or clinical evidence of malignancy has been demonstrated up to 8 years after normal-mode ruby laser treatment.23,58 However, since congenital nevi have the potential to transform into malignant melanoma, and residual nevus cells persist in the dermis after laser treatment, cautious long-term follow-up of nevi treated with lasers is required.
Both continuous wave lasers60,61 and the QSRL62 have been used to treat lentigo maligna. Nonetheless, there are several reports of lentigo maligna recurring following laser treatment, probably due to persistence of melanocytes within deeper adnexal structures.63,64 Laser treatment of lentigo maligna should be reserved for extreme situations where surgical excision is not feasible due to large lesion size, advanced patient age, or underlying medical condition. Close follow-up to detect any early recurrence is critical.
Nevus of Ota
Nevus of Ota (also known as oculodermal melanoma or nevus fuscoceruleus ophthalmomaxillaris) is a mottled, blue-grey macule that is usually located unilaterally within the distribution of the first and second branches of the trigeminal nerve. Lesions usually occur in a 5:1 female to male ratio. Asians are most commonly affected, with an incidence of 1 in 500 reported in Japan.65 Nevus of Ota is congenital in about 50% of cases, with others appearing by the second decade of life. Nevus of Ota may affect mucosal surfaces such as cornea, sclera, nasal and buccal mucosa, and tympanic membrane. Histologically, elongated dendritic melanocytes are scattered within the upper dermis. The occurrence of melanoma within nevus of Ota has been reported66 but is rare.
Q-switched lasers are extremely helpful in treating Nevus of Ota. The degree of lightening is usually directly proportional to the number of treatments performed. Lightening of 70% or more has been reported in the majority of patients treated four or five times with the QSRL57 (Fig. 12). Post-treatment dyspigmentation occurs occasionally, although textural change has not been reported. Post-treatment biopsies have revealed disintegration of melanocytes up to a depth of 1.5 mm from the skin surface.57 While the QSRL has been most widely used,57,67–70the Q-switched alexandrite71 and Nd:YAG25 lasers seem as effective. Large-scale comparative trials between these lasers have not been performed.
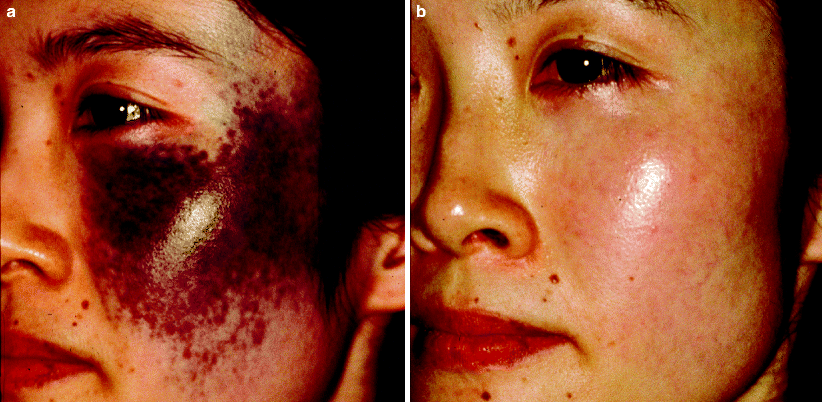
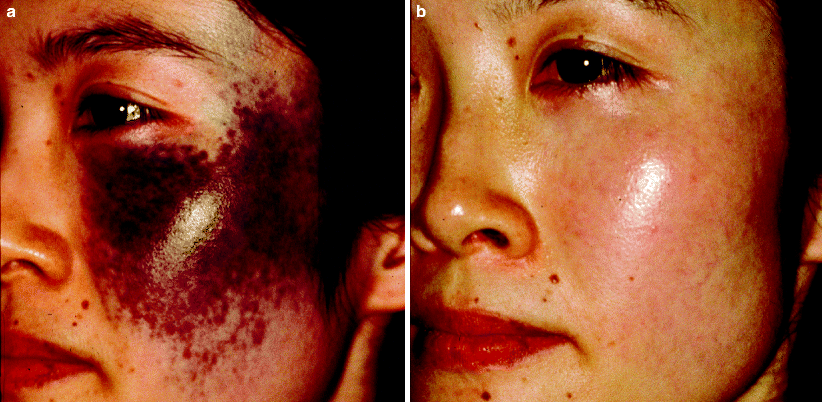
Fig. 12
(a) A patient with a dark nevus of Ota extending over a significant portion of the face prior to treatment. (b) After a series of treatments with the Q-switched ruby laser, there is impressive lightening of the lesion with no textural change
Acquired nevus of Ota-like macules (also known as Hori’s nevus) differ from the classic nevus of Ota in that they are bilateral, spare mucosa, and occur later in life. Various Q-switched lasers have been reported effective in treating nevus of Ota-like macules.72–74 Mauskiatti et al. demonstrated greater clearing of bilateral nevus of Ota like macules with a combination carbon dioxide (CO2) and Q-switched ruby laser (QSRL) treatment than with QSRL alone.75 Laser treatment of any pigmented lesion is often complicated by post-inflammatory hyperpigmentation. A recent study of Q-switched Nd:YAG treatment for acquired nevus of Ota-like macules suggests that epidermal cooling may be associated with an increased risk of post-inflammatory hyperpigmentation.76
Melasma and Post-inflammatory Hyperpigmentation
Melasma is a common acquired hyperpigmentation, most often affecting adult females with skin type III or higher. It occurs as brown to blue-grey macules most frequently on the cheeks, forehead, upper lip, nose, and chin. It is associated with sun exposure, pregnancy, and use of oral contraceptives, although it can also be seen in patients without any predisposing factor. Melasma can have increased melanin in either the epidermis, dermis or both. Initial management consists of discontinuing any oral contraceptives or hormonal replacement, and strict sun avoidance. Hydroquinone alone or in combination with topical corticosteroids or retinoids is the mainstay of treatment. Azeleic acid, kojic acid, and superficial chemical peels also provide some benefit. Melasma with dermal melanin is the most difficult to treat. Post inflammatory hyperpigmentation has a similar clinical and histology morphology as melasma, but develops following cutaneous injury or inflammatory process. Studies have shown that Q-switched lasers are largely ineffective in the treatment of melasma and post-inflammatory hyperpigmentation.25,77 Q-switched laser treatment may actually cause an increase in dermal melanophages and worsening of hyperpigmentation. Carbon dioxide78 or erbium:YAG79 laser resurfacing provides an alternative treatment modality for melasma, but post-inflammatory hyperpigmentation is extremely frequent in the postoperative period and as a result these treatment modalities are not recommended. Some of the newer laser technologies, such as the fractionated erbium fiber laser (Fraxel), can be useful in treating melasma.80,81 A study of ten patients showed 75–100% clearing of melasma in 60% of patients treated with the fractionated erbium fiber laser (Fraxel).82 These patients were treated at a low fluence but a high density of microthermal zones. Fractionated laser treatment may work by expelling columns of microscopic epidermal debris that contains melanin.
Infraorbital hyperpigmentation (dark circles) may result from a variety of causes, including dermal melanin deposition, post-inflammatory hyperpigmentation from atopic or allergic contact dermatitis, prominent superficial blood vessels, and shadowing from skin laxity and infraorbital swelling71 The QSRL has been reported to effectively treat infraorbital hyperpigmentation when due to deposition of dermal melanin.83




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








