Cutaneous wound healing is divided into three phases: the inflammatory phase, the proliferative phase, and the remodeling phase
Inflammatory phase starts with tissue injury which causes extravasation of platelets, neutrophils, and monocytes to the site of injury. These cells release a variety of cytokines, inducing epithelial cell migration and proliferation
In the proliferative phase, keratinocytes and fibroblasts proliferate and migrate to the wound bed in order to close the defect
In the remodeling phase, remodeling of the collagen into a more organized structure occurs in order to increase the wound’s tensile strength
The wound healing process can be applied to both acute and chronic wounds. Acute wounds are generally less than 8 weeks, and usually result in a sustained restoration of anatomic and functional integrity. Chronic wounds are defined as wounds that have failed to proceed through the usual stepwise fashion. Lasers are used for healing of both acute and chronic wounds
Understanding the processes involved in wound repair is a prerequisite to maximize our knowledge regarding the use of lasers for wound healing. Cutaneous wound healing involves the complex interaction of several types of cells, their cytokines or mediators, and the extracellular matrix. After cutaneous injury, a cascade of events is observed, which mediates tissue repair and eventually the reestablishment of the barrier function of the skin. Tissue repair is divided into three phases: the inflammatory phase, the proliferative phase, and the remodeling phase (Fig. 1).5
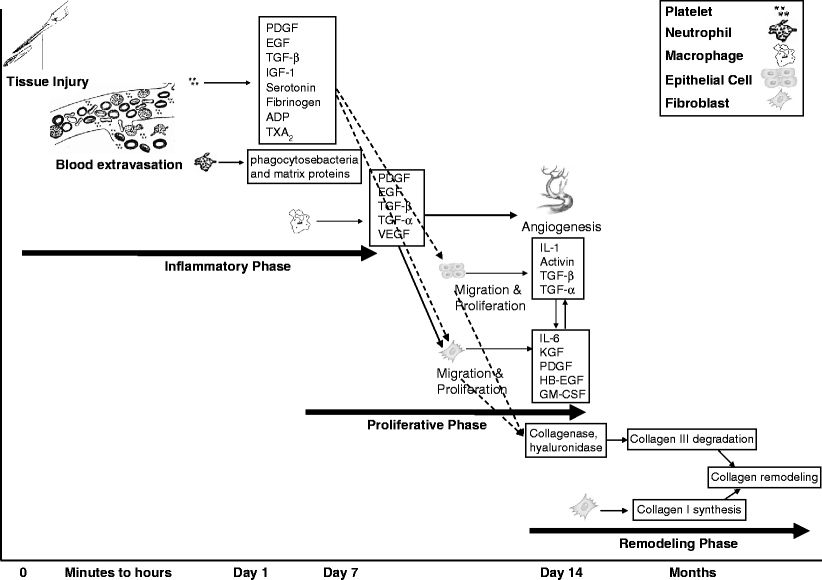

Fig. 1
Phases of wound healing. The inflammatory phase starts within minutes after tissue injury with extravasation of blood followed by activation of platelets, monocytes and macrophages, release of mediators and cytokines. These cytokines induce the proliferative phase by activating keratinocyte and fibroblast proliferation and migration, as well as release of a variety of growth factors involve in angiogenesis and granulation tissue formation. The last phase of wound healing is remodeling via replacing collagen III by collagen I. IL interleukin, KGF keratinocyte growth factor, FGF fibroblast growth factor, VEGF vascular endothelial growth factor, PDGF platelet-derived growth factor, EGF epidermal growth factor, HB-EGF heparin binding EGF, TGF-α transforming growth factor-alfa, TGF-β transforming growth factor-beta, IGF-1 insulin-like growth factor-1, GM-CSF granulocyte-macrophage colony stimulating factor, ADP adenosine diphosphate, TXA2 thromboxane A2
Inflammatory Phase
The initial event in tissue injury is the damage to endothelial cells and blood vessels. This causes extravasation of blood into the wound and collagen exposure which leads to blood clotting, platelet aggregation and activation, as well as migration of neutrophils and monocytes (and subsequently macrophages) to the site of injury. Activated platelets release a variety of mediators (Fig. 1) which initiate the wound healing cascade by attracting and activating fibroblasts, endothelial cells and macrophages. Neutrophils, once in the wound environment, phagocytose bacteria and matrix proteins. Later in the inflammatory phase, monocytes and macrophages become the dominant figures, and release a variety of cytokines, inducing epithelial cell migration and proliferation as well as matrix production.5–7
Proliferative Phase
This phase involves the creation of a permeability barrier as well as the establishment of an appropriate blood supply and reinforcement of the injured tissue. Keratinocytes and fibroblasts proliferate and migrate to the wound bed. Fibroblast proliferation and migration are modulated by PDGF, EGF, TGF-α, TGF-β and FGF. Macrophages play a key role in initiating fibroblast proliferation and migration. When the number of macrophages begins to diminish, fibroblasts and keratinocytes are the main source of the growth factors. The interplay of keratinocytes with fibroblasts gradually shifts the microenvironment away from an inflammatory to a synthesis-driven granulation tissue.
In the granulation tissue, mesenchymal cells become maximally activated, proliferate, and synthesize huge amounts of extracellular matrix which supports the developing capillary loops. Keratinocytes proliferate and migrate over the provisional matrix of the underlying granulation tissue, eventually closing the defect.5 ’ 8 ’ 9
Remodeling Phase
In this phase, remodeling of the collagen into a more organized structure occurs in order to increase the wound’s tensile strength. The type III collagen of the granulation tissue is replaced by type I collagen through a tightly controlled synthesis of new collagen and lysis of old until the normal skin ratio of 4:1 for type I collagen to type III collagen is present. In addition, the composition of other matrix material such as water, fibronectin, hyaluronic acid, and proteoglycans changes over the period of a year or more.5 ’ 10
Acute Versus Chronic Ulcers
The wound healing process can be applied to both acute and chronic wounds. Acute wounds are generally less than 8 weeks, and usually result in a sustained restoration of anatomic and functional integrity. Chronic wounds are defined as wounds that have failed to proceed through the usual stepwise fashion. As a result, the healing process is prolonged and incomplete, with lack of restoration of integrity.11 A large number of factors can impede wound healing and may predispose a patient to the development of a chronic wound. These include both local factors (wound infection, tissue hypoxia, repeated trauma, the presence of debris and necrotic tissue) and systemic causes (diabetes mellitus, malnutrition, immunodeficiency, and the use of certain medications).12 ’ 13
Lasers for Wound Healing
The use of lasers for wound healing has been focused in two fields: lasers to augment the healing of acute wounds (e.g., tissue welding, tissue soldering), and lasers for chronic wounds (e.g., low intensity laser devices) |
The use of laser energy for wound healing was proposed more than 35 years ago.14 It was first suggested for bonding skin incisions, and termed “laser welding.” Interest in the efficacy of lasers as a noninvasive tool for treatment of all types of wounds soon grew among researchers in both animal models15 and clinical studies.16 The concept that surgeons can replace their scalpels and tedious suturing techniques with a simple, non–operator-dependent, safe, and rapid technique, has inspired many investigators to experiment on different laser systems.17 The areas of research can be divided in two major groups: lasers to augment the healing of acute wounds (tissue welding, tissue soldering, etc. see below), and lasers for chronic wound (e.g., low intensity laser devices, see below). Although these two groups of lasers share many similarities, there are differences in their mechanism of action, laser systems, laser parameters, etc. These factors will be discussed in this chapter.
Lasers for Acute Wounds
The main techniques of laser-assisted wound closure of acute wounds are: simple tissue welding, tissue soldering, dye-enhanced tissue welding, and addition of growth factors |
The potential advantages of laser-assisted tissue bonding over conventional methods include increased immediate wound strength, fluid-tight closure, decreased operative repair time, reduced probability of infection and bleeding, and improved cosmetic results. However, lasers have disadvantages such as their high cost, risk of dehiscence, risk of thermal damage, and inconsistency of results |
The exact mechanism involved in laser-assisted wound closure is not completely understood. The heat produced by laser energy in the tissue causes collagen fibers to lose their triple helix structure and become fused, intertwined, swollen, and dissolved |
Interest for tissue welding for closure of acute wounds first came out of early experiences with the use of electrocautery energy.18 Later, laser energy was introduced for vascular anastomosis and then for other types of acute wounds. After the introduction of laser-assisted wound closure, it was rapidly evident that welding of skin was difficult. In fact, the initial tensile strength of the wound was weak compared with conventional sutures in the first few days post incision.15 ’ 19 ’ 20 However, the wound healing process was generally accelerated, and the cosmetic aspect of the scar was improved. In order to enhance the tensile strength and minimize the thermal damage, various improvements have been suggested. The main techniques are simple tissue welding, tissue soldering, dye-enhanced tissue welding, and addition of growth factors (Table 1).
Table 1
Lasers commonly used in acute wound healing
Technique | Laser system + solder/dye |
|---|---|
Tissue welding | CO2 |
Argon | |
Nd:YAG | |
Diode | |
Tissue soldering | Diode + albumin-genipin |
Diode + methylene blue | |
Diode + albumin | |
Diode + fibrinogen | |
CO2 + albumin | |
Nd:YAG + albumin | |
Argon + fibrinogen | |
Dye-enhanced | Alexandrite + indocyanine green |
Argon + fluorescein isothiocyanate | |
Diode + indocyanine green | |
Diode + gold nanoshells |
Tissue welding: The first method introduced for laser-assisted wound closure was “tissue welding.” The principle of laser-assisted tissue welding is based on the heat produced by the laser irradiation. The increased temperature in the skin causes collagen denaturation and the crosslinking of fibrils.21 It is crucial to estimate the optimal photonic energy that is to be delivered to tissue. In this respect, major determining factors are laser wavelength, power, exposure time, and mode of operations (continuous wave or pulsed). For this reason, various types of laser systems were investigated (Table 1).15 ’ 22–24 The first successful use of a laser in tissue welding was in 1979 when ND:YAG was used to repair incisions made in blood vessels of a rat.25 Later, tissue welding was successfully performed for skin closure as well as for anastomosis of other tissues.15 Despite progresses made in tissue welding, surgeons still do not embrace this new laser technology. The main reasons can be summarized in three main drawbacks of laser welding: (1) low tensile strength during the first few days, (2) noticeable thermal damage, (3) inconsistency of results.26
Tissue soldering: Laser-assisted tissue soldering uses an additional component known as a “solder” for better wound closure. The solder (bovine albumin, human albumin, blood, etc.) absorbs the laser energy, coagulates, and as a result, enhances the tensile strength while minimizing the thermal damage of the surrounding tissue.17 ’ 27 Laser-assisted tissue soldering has been carried out using two types of lasers: lasers such as Nd:YAG and GaAs, whose radiation penetrates deep into tissue28; and lasers such as CO2, whose radiation is highly absorbed by surface tissue.19 A variety of solders have also been studied (Table 1). Albumin as a solder, was introduced in 1988, and showed to be promising in studies with CO2, diode and Nd:YAG lasers.15 ’ 29–31 Other solders such as fibrinogen,32 Albumin-genipin,33 and methylene blue34 have also been suggested. Again, the major drawback of this technique was the weak tensile strength of the repairs due to the decreased solubility of the partially denatured solder. To overcome this problem, “2-layer” soldering was developed. In “2-layer” soldering, the layer in contact with tissue absorbs the laser and bonds to tissue while the second layer provides cohesive strength and flexibility. The main limitation of this method is lack of flexibility of bonded tissue.35
Dye-enhanced tissue welding: The concept of using a topical tissue-staining dye to facilitate selective delivery of laser energy by the target tissue has been postulated to improve tissue welding. A nontoxic dye that is strongly absorbed by a specific laser wavelength can serve to confine photon absorption and the resultant thermal energy to the weld site. A variety of combinations of dyes and lasers have been studied with variable success rates.16 ’ 32 ’ 36 ’ 37 It seems that combination of indocyanine green with either pulsed alexandrite or pulsed diode laser is superior to other dye-enhanced tissue welding techniques. Nonetheless, it is worth noting that very limited clinical data have been available yet that confirm the clinical value of dye-enhanced tissue welding.
Nanoshells are a new class of nanoparticles consisting of a dielectric core surrounded by a thin metal shell. Use of gold nanoshells in conjunction with near infrared light has recently been suggested as a means of dye-enhanced tissue welding. Application of lasers at wavelengths within the near infrared, between approximately 650 and 900 nm, where tissue components have minimal absorption, decreases the chance of widespread thermal damage and improves penetration depth.38 The use of nanoshells has several advantages over indocyanine green. For example, the small size of nanoshells reduces diffusion from the site of treatment and concentrates heating at the interface to be welded. Also, they are less photosensitive hydrolytically sensitive and susceptible to photobleaching in the presence of light compared to indocyanine green.
Addition of growth factors: Attempts have been made to use recombinant growth factors, as an adjunct to laser-assisted tissue soldering to accelerate wound healing. A variety of growth factors such as HB-EGF, FGF, TGF-β, etc. have been studied. The result of an animal study by Poppas and colleagues showed that addition of TGF-β to the solder (albumin in their study) increases the tensile strength of the wound by more than 50%. Using this technique, it is imperative to maintain a predetermined tissue temperature in order to prevent thermal degradation of growth factors.39
Lasers Versus Conventional Methods of Acute Wound Closure
Conventional techniques for tissue bonding (sutures, staples, and adhesives) are highly reliable procedures that have proven themselves over the years to be good clinical practice. Sutures have been successfully used for centuries. They are inexpensive, flexible, reliable, and readily available.15 However, they are not the perfect technique due to several reasons (Table 2). Since sutures cause trauma to the skin, and introduce a foreign body, they can result in inflammation, granuloma formation, and scarring. Many technical factors such as position of the needle in the holder, the slope of the tissue at needle entrance, suture spacing, knot tension, and choice of suture material can affect wound healing.23 ’ 40 Staples are another mean of wound closure which share many common characteristics with sutures. However, they are faster and more uniform than sutures. Their main disadvantage is that they come in predetermined size which precludes their use in some anatomical sites. Adhesives are a clean, fast, non-operator-dependant, painless method of wound closure. They are an excellent “no needle” alternative in pediatric patients. However, for most applications, they have not been able to provide adequate strength.15 ’ 41 ’ 42
Table 2
Advantages and disadvantages of different methods of wound closure
Advantage | Disadvantage | |
|---|---|---|
Suture | Reliable | Cause trauma to the tissue |
Flexible | Time consuming | |
Inexpensive | Operator dependant | |
Available | Introduce foreign body | |
No immediate watertight closure | ||
Risk of needle-stick | ||
Risk of infection (due to lack of sealing) | ||
Need suture removal | ||
Staple | Reliable | Cause trauma to the tissue |
Available | Inflexible (predetermined size) | |
Relatively quick | Introduce foreign body | |
Not operator dependant | No immediate watertight closure | |
Risk of needle-stick | ||
Risk of infection (due to lack of sealing) | ||
Need staple removal | ||
Adhesive | Immediate watertight closure | Expensive (controversial) |
Painless | Does not provide hemostasis | |
No trauma to tissue | Introduce foreign body | |
“No needle” procedure | (Possible) need for subcutaneous sutures | |
Less risk of infection | Risk of tissue reactivity | |
No need for removal | Not flexible (comparing to sutures) | |
Fast | ||
Laser | Immediate watertight closure | Expensive |
better scar | Not readily available | |
“No needle” procedure | Foreign body (Soldering, dye-enhanced) | |
Less risk of infection | Risk of dehiscence | |
No need for removal | Complicated (many parameters to consider) | |
Fast | Risk of thermal damage | |
Dynamic effects (may increase growth factors) | Inconsistent results |
As shown in detail in this chapter, laser-assisted tissue bonding can transcend the limitations of conventional methods in many aspects. Their potential advantages over conventional methods include increased immediate wound strength, fluid-tight closure, decreased operative repair time, reduced probability of infection and bleeding, and improved cosmetic results. However, there have been several obstacles which prevented physicians from using laser welding clinically. These included collateral thermal injuries, inconsistency of results, and a lack of understanding of the exact mechanism by which laser irradiation induces tissue bonding. In addition, there are many parameters that need to be optimized in the welding process. These parameters include wavelength, fluence, pulse duration, repetition rate, irradiation time, spot size, and solder selection. Indeed, the parameter window for optimum tissue bonding is very small. All parameters should be chosen appropriately to provide enough heat for denaturation and crosslinking of collagen fibers, but not to the level of tissue necrosis and sloughing of wound edges. What makes the use of laser even more complicated is the fact that energy levels and exposure times that may work very well with certain tissues may not be the best for other situations.15 ’ 17 As we discuss later in this chapter, several thermal feedback systems have been suggested to overcome the above limitations.
Mechanism of Laser-Assisted Wound Bonding
The exact mechanism involved in laser-assisted wound closure is not completely understood. Nonetheless, what is commonly believed is that tissue bonding occurs mainly due to the thermal effect of laser. The heat produced by laser energy in the tissue causes collagen fibers to lose their triple helix structure and become fused, intertwined, swollen, and dissolved. This generates a coagulum that serves both as a coating for sealing the wound and as a sophisticated scaffold for re-colonization of cells, as in the case of re-epithelialization.24 ’ 43 ’ 44




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








