Fig. 3.1
On the left. Basic elements of the breast: (1) glandular component including segmental ducts (and then collecting major ducts) with terminal ductal-lobular units (TDLU); (2) stromal component with Cooper’s ligaments of the breast which form fibrosepta in the stroma and provide support for the breast parenchyma; (3) adipose component with subcutaneous fat and adipose tissue distributed around the lobules of the gland which gives its smooth contour and accounts for most of its mass. On average, in premenopausal women, the glandular component varies from 15 to 20 %, while in menopausal woman, it decreases to 2–5 %. On the right. Anatomy of terminal ductal-lobular units (TDLUs), a combination of extralobular terminal ducts and lobules. Lobules are composed by intralobular terminal ducts and acini or ductules (for a detail, see Fig. 9.8).
Macroscopic functional unit (segment, lobe and lobules). The fibroglandular tissue of the breast is divided into 12–20 segments that converge at the nipple in an unevenly radial arrangement. These segments are not always uniformly distributed around the breast. The upper half of the breast, particularly the upper outer quadrant, tends to contain more glandular tissue than does the remainder of the breast. Each segment contains a lobe made of 20–40 lobules, each consisting of more or less 100 alveoli. A 2-mm duct drains each segment into subareolar lactiferous sinus (or ampulla lactifera) of 5–8 mm in diameter. About 10 major collecting ducts then open at the nipple.
Microscopic functional unit (TDLU). The fundamental glandular unit of the breast, and biologically its most actively proliferating part, is the terminal ductal-lobular unit (TDLU). Each of the lobes in the breast contains thousands of TDLUs, which form the functional secretory unit. During pregnancy and lactation, the epithelial cells of the terminal ducts and lobules undergo secretory changes, and the units produce milk, which drains via the branching segmental ducts to their ampullae at the surface of the nipple. Hence, the lobules are referred to as acini during pregnancy and lactation.
The TDLU is complex and consists of the extralobular and intralobular terminal ducts and the blindly ending lobules. The epithelial lining of the lobule consists of superficial cells (luminal A) that are involved in milk synthesis. The B cells (basal) have stem cell activity. Except for the terminal portion of the collecting ducts, low-columnar to cuboidal epithelium lines almost the entire duct system of the breast, including the segmental ducts, subsegmental ducts, terminal ducts and acini.
An outer layer of myoepithelial cells, which contains contractile fibres, facilitates milk secretion via their contractile property, which is largely under the influence of oxytocin, primarily responsible for the release of milk, a phenomenon called milk letdown. The myoepithelial cell layer is generally regarded as being spindle-shaped (see also Fig. 9.5), and it extends from collecting ducts to the tip of the acini.
The basement membrane, composed of a relatively attenuated basal lamina, lies immediately outside of the myoepithelial cell layer and divides the glands from the stroma that lies beyond the basement membrane.
The mammary ducts and lobules are embedded within a variable fibrous and fatty stroma. The relative portions of glands and fibrous and adipose tissue vary with age and body habitus; however, stromal tissues make up the bulk of the breast in adult non-lactating and non-pregnant women. Adipose tissue is typically present in the extralobular stroma and not in the intralobular stroma among lobules (at least not until atrophy ensues). The fibrous tissue assists in the mechanical coherence of the gland.
Most disease of the breast arises from the TDLUs, including cysts, which may be the consequence of the unfolding of the terminal ducts and lobular units. Indeed, the only common lesion believed to be strictly of ductal origin may be the larger solitary intraductal papilloma. The sites of origin of common diseases of the breast are represented on Fig. 8.1.
Superficial lymphatic drainage. The superficial plexus of lymphatic vessels exists throughout the entire body surface. These vessels are valveless, allowing lymph to flow in any direction, although it does so sluggishly. The subepithelial plexus connects to subdermal lymphatic vessels by vertical lymphatics. The subdermal vessels do have valves. Thus, lymph flows unidirectionally from the superficial to the deep plexus. In the breast, the subepithelial and subdermal plexuses are confluent with the subareolar plexus. Also draining into the subareolar plexus are the fine lymphatics of the lactiferous ducts and the lymphatics of the areola and nipple.
Deep lymphatic drainage. From the deep lymphatics, the lymph flow moves centrifugally towards the axillary and internal mammary lymph nodes. About 10 % of the lymph from the breast goes to the intramammary chain, which can come from all quadrants of the breast, not just the inner quadrants. The other 70–90 % of lymph flows to the axillary lymph nodes.
Boundaries of the axilla. The axilla is typically thought of as a four-walled pyramid that sits between the upper arm and the chest. The dome-shaped base of the pyramid is the armpit, made of the axillary fascia and the skin. The anterior wall is the pectoralis major and minor muscles. The posterior wall is the subscapularis muscle (and to a lesser extent the teres major and latissimus dorsi muscles and tendons). The medial wall is the serratus anterior muscle. The lateral wall is a thin band of humerus between the insertions of the muscles of the anterior and posterior walls.
Structures of the axilla. Coming through the apex of the pyramid are the great vessels and nerves of the upper extremity, enclosed within a layer of fascia, the axillary sheath, a dense connective tissue that gradually disappears as the nerves and vessels begin to branch. The axilla is enveloped in fascia. The most significant fascia is the clavipectoral fascia that extends from the clavicle towards the floor of the axilla (the axillary fascia). The lower portion of the clavipectoral fascia is sometimes called the suspensory ligament of the axilla or the coracoaxillary fascia.
Axillary lymph nodes. The lymph nodes of the axilla are anatomically categorized by several groups (Fig. 3.2): posterior or subscapular; anterior, laterally to pectoralis; lateral, facing the humeral head; central; subclavicular, posterior to pectoralis minor; apical; and interpectoral (or Rotter’s nodes).
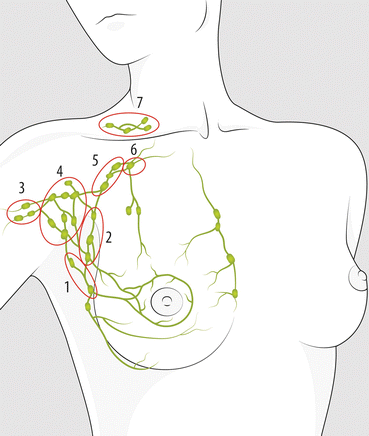

Fig. 3.2
Lymph drainage and lymph node groups of the axilla and clavicular fossa: 1 posterior or subscapular, 2 anterior or lateral pectoral, 3 lateral, facing the humeral head, 4 central, 5 subclavicular, posterior to pectoralis minor, 6 apical, 7 clavicular groups. Seventy to ninety percent of the lymphatic drainage from the breast takes place into the axillary nodes. Lymph from three groups of axillary nodes (posterior, anterior and lateral) drains into the main group (central) of nodes that is high in the axilla. All nodes are in continuity with each other, but an arbitrary division in three axillary node levels is considered useful in surgical or pathology reports. Level I (low axilla or external nodes) includes 1, 2, 3 and 4 groups. Level II (midaxilla) includes subclavicular group, posterior to pectoralis minor. Level III (apical axilla) includes an apical group of few nodes superomedial to the pectoralis minor. Intramammary nodes and interpectoral nodes (Rotter’s nodes) are included in level I
Surgically, axillary lymph nodes are categorized in three levels based in their relation to the pectoralis minor muscle: the level I lymph nodes are lateral or below the lower border of the pectoralis minor muscle and include the external mammary, axillary vein and scapular lymph node groups, the level II lymph nodes are located just beneath the muscle, and the level III nodes (or apical) are medial to the medial border of the pectoralis minor.
3.1.2 Development of the Breast
The breast undergoes multiple changes throughout life, from intrauterine life to senescence. Although the majority of growth occurs with puberty, the development and differentiation of the breast are truly completed by the end of the first term of pregnancy with the postpartum lactation of the adult female. This is relevant to the development of cancer, because BC risk is somewhat inversely related to the age at which pregnancy first occurs. It is possible that this is secondary to an increased risk of carcinogenesis when the pre-parity, undifferentiated and proliferating mammary epithelium is exposed to carcinogens, as compared to the effect of these same carcinogens on the differentiated breast.
At birth, after the transient secretion stimulated by prolactin production in the neonate, the mammary glands, with their relatively simple architecture, remain quiescent until puberty. During this period, the supporting stromal structures and ducts enlarge in proportion to the increase in body size of the individual, but no lobular development occurs.
PUBERTY – The rapid growth that occurs at the onset of puberty is primarily from deposition of fat and development of periductal connective tissue, but elongation and thickening of the ductal system also occur at this stage. Under the influence of gonadotropin-releasing hormone from the hypothalamus, puberty begins in children between 8 and 12–13 years of age. This leads to the release of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) from the pituitary gland, resulting in maturation of the ovarian follicles and the secretion of oestrogens. As puberty begins, the circulating oestrogen causes the ductal epithelium and surrounding stroma to grow. These ducts begin to extend into the superficial pectoral fascia and arborize within the supporting stroma to form collecting ducts and terminal ductal-lobular units. These ultimately form buds that precede further breast lobules. Surrounding the ducts, vascularity increases and connective tissues increase in volume and elasticity, replacing adipose tissue and providing support for the developing ducts.
Ductal growth occurs under the influence of circulating oestrogens, growth hormone and prolactin but is independent of progesterone.
Although there are many ways one can define the stages of breast development from puberty to adulthood, the most commonly used system is the Tanner phases, which is based also on the external appearance of the breast, listed to follow.
Tanner I glandular tissue: areola follows the skin contours of the chest (prepubertal, usually age 10 and younger).
Tanner II: bud forms, with small area of surrounding glandular tissue; areola begins to widen (age 10–11.5).
Tanner III: breast begins to become more elevated and extends beyond the borders of the areola, which continues to widen but remains in contour with surrounding breast (age 11.5–13).
Tanner IV: increased breast size and elevation; areola and papilla form a secondary mound projecting from the contour of the surrounding breast (age 13–15).
Tanner V: breast reaches final adult size; areola returns to contour of the surrounding breast, with a projecting central papilla (age >15).
The development initiated at the onset of puberty is generally complete by age 20 years, but approximately a year or 2 following menarche, the breasts acquire their mature macroscopic structure (Tanner V).
PREGNANCY – In the pregnant patient, there is marked growth of the ducts, lobules and alveoli under the influence of luteal and placental sex steroids and prolactin.
In the first trimester, during the first 3–4 weeks of pregnancy, under the influence of oestrogen, there is growth and branching of the ducts, as well as increased lobule formation. By the second month, the breasts have enlarged dramatically. The superficial veins dilate and there is increased pigmentation of the nipple-areolar complex. The breasts become tender and the nipples become sore. This can begin just a few weeks after conception.
Oestrogen and progesterone prohibit the hypothalamus from producing prolactin-inhibiting factor (PIF). With this influence gone, prolactin is released and this continues progressively during pregnancy, although increase in prolactin levels is slow during the first trimester.
In the second trimester of pregnancy, the effects of progesterone cause the lobular formation to exceed the ductal sprouting. During this time, prolactin levels continue to rise and by the third trimester, blood levels of prolactin are three to five times higher than normal. At this point, the alveoli contain colostrum but no fat. The breast continues to enlarge, but this is not due to epithelial proliferation but rather the filling of the alveoli with colostrum as well as the hypertrophy of myoepithelial cells.
In the third trimester of pregnancy, the stroma surrounding the lobules diminishes to make room for the hypertrophied lobules. As pregnancy continues, colostrum composed of desquamated epithelial cells and fluid accumulates. This is released in the immediate postpartum period.
LACTOGENESIS – In pregnancy, prolactin is being produced, starting during the eighth week of pregnancy and increasing until birth. During that time, the high levels of oestrogen and progesterone block the prolactin receptors and inhibit milk production. After birth, there is a decline in the serum levels of oestrogen and progesterone over several days. This removes the inhibition on milk production and lactogenesis begins.
Prolactin is one of two hormones responsible for milk production, with the other being oxytocin. Prolactin levels were increasing until birth, and after delivery begin to decline. If the mother is not nursing, the prolactin levels will drop slowly in about 14 days. In the nursing mother, prolactin will also drop, but much more slowly, dependent on the time that the infant nurses. Prolactin drives the synthesis and secretion of milk into the alveolar spaces, then the myoepithelial cells contract, and the milk passes through the ductal system and out of the breast.
Oxytocin is the second hormone responsible for milk production and delivery. When an infant suckles at the mother’s breast, this causes the increase in both prolactin, to stimulate the production, and oxytocin, to increase milk delivery.
During the first few days after delivery, the body does not produce milk but rather a colostrum. This is high in immunoglobulins, which help protect the infant against infections at a time when the infant’s own immune system has not fully developed. Colostrum may help decrease the infant’s chances of developing asthma and other allergies. When breastfeeding stops, it may take several months for milk production to completely stop. The breasts usually return to their previous size, although they may be smaller after breastfeeding is completed.
Postlactational involution starts on weaning and is initiated by local mechanical factors causing alveolar distension and capillary obstruction. The one-layer secretory alveolar cells regress and reform the two-layered epithelium characteristic of the resting breast. This process is facilitated by cell death and phagocytosis performed by invasion of the alveoli by histiocytes. A lymphocytic infiltrate is also characteristic, but connective tissue regression is limited. The branching alveolar structures become fewer in number but the ductular structure remains mostly intact – this is the fundamental difference between postlactational and postmenopausal involution, where both lobules and ductules are reduced in number. The ducts become smaller although some secretion remains persistently in the duct lumen in the postlactational breast and can be aspirated or expressed from the nipple in most parous women.
MENOPAUSE – During and after menopause, the altered hormonal environment leads to a senescent state, with involution of the glandular component and replacement with connective tissue and fat. The involutive process can be divided into a preclimacteric phase starting at about the age of 35 and a postmenopausal phase starting at the time of the menopause, in late 40 and early 50 years of age. The predominant feature is regression of the glandular epithelium and adjacent connective tissue with gradual replacement by fat.
In the preclimacteric phase, there is a gradual loss of lobules and infiltration by round cells and the specialized loose connective tissue around the lobules changes into dense collagen. In the postmenopausal phase, the typical outline of a lobule is lost and is replaced by dense collagen containing a compressed epithelial remnant. Lobular involution may proceed to formation of microcysts, which may be mistaken for cystic disease microscopically. The essential difference between the two conditions is the preservation of the specialized lobular stroma in the former. The loss of strength of the connective tissue results in an increase in size and sag to the breasts. However, these changes of atrophy are variable and incomplete. Some women in their 60s and 70s still have a lobular appearance similar to a premenopausal state.
Hormone therapy may delay postmenopausal changes in the breast and mimic a more active physiologic or premenopausal state (i.e. cyclic tenderness due to increased nodularity). It would therefore seem logical that fibrocystic changes should resolve with menopause, but this is not always the case. For some women, the breasts can become more tender with menopause, with an increase in nodularity or cysts.
3.1.3 Clinical Significance of Constitutional Patterns
Breast density is not an intuitive concept and has little to do with breast size and, in some cases, with age. Though it can be influenced by lifestyle factors, twin studies show that the underlying causes of breast density are mostly inherited. Also, in common practice, constitutional patterns of the breast are similar among first-degree member of family, independently from their body structures.
Higher breast density is more common in some ethnic groups, including white women. It is also more common in younger women, beginning when hormones kick in during puberty and continuing through the childbearing years. Breast density decreases during menopause in a process called breast involution, where the milk glands and ducts atrophy and connective tissue disappears. But in some women, these tissues persist into older age.
The breast composition and relative amount of glandular tissue, connective tissue and fat is well documented in the white (glandular) and black (fat) areas on mammograms. Different methods of estimating the proportion of white area on the mammogram exist and vary from the perception of the radiologist to using a software programme to outline the white area and compare it to the total breast area.
The BI-RADS measuring system involves a radiologist scoring a mammogram in one of four categories according to the extent of contrast within the outline of the breast: x-rays pass easily through fatty tissue, which shows up as darker areas on the image, but are blocked – and thus appear white – by milk ducts, lobes and the web of connective tissue that tethers everything together. Studies have shown that women who have extremely dense breasts have a three- to fivefold increased risk of breast cancer compared with women who have mostly fatty breasts (see Sect. 2.1).
Both in clinical and radiological exam, the major concern is whether dense breasts have a masking effect, where the lack of contrast between normal and pathological tissue in dense breasts makes it difficult to identify abnormal clinical densities and radiological opacities (see Sect. 5.1).
Furthermore, from the clinical point of view, the possible extensions of glandular tissue at the periphery of the gland (axillary, subclavian prolongation and extension over normal medial and inferior boundaries) must be taken into account for justifying some unusual clinical manifestations. The pathology of the extended parenchyma or accessory glands is similar to that of the normal mammary gland, with equal possibility of cancerization.
3.2 Congenital Breast Deformities
Clinical Practice Points
Congenital malformations of the breast, relating mainly to absence, hypoplasia or ectopia, are sometimes part of wider congenital syndromes, with particular tendency to affect the urinary tract or limb girdles.
The foetal milk line is not as extensive in humans as in some animals, so most ectopic tissue is found between the axilla and epigastrium.
Axillary accessory breasts can be subject to all varieties of pathology seen in the breast proper, so axillary masses should be assessed individually, as well as in association with any breast mass.
Some developmental abnormalities occur so early that are believed to be congenital when they actually are due to abnormal development of the stromal or lobular component.
Accurate counselling may be necessary to alleviate the sense of deformity and unattractiveness if present. In teenage years, proper timing of surgical intervention is necessary to optimize functional, psychological and aesthetic outcomes.
The mammary malformations are numerous and there are several classifications, most of which descriptive. These malformations generally fall into two categories: the presence of supernumerary breast tissue and the absence or underdevelopment of breast tissue. In a more general framework, which also includes pathological manifestations as altered development, we prefer to distinguish them as follows:
Deformities regarding the number and location
Deformities regarding the shape (breast hamartoma and tuberous breast)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








