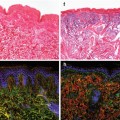
Fig. 4.1
The Drosophila male stem cell niche: (a) schematic representation of the stem cell niche at the apical tip of the testis. Hub cells (red). Germline stem cell (GSC)/progenitor gonialblast (GB)/spermatogonia cells (green). Cyst stem cell (CySC, light gray), cyst cells (dark gray). (b) Dual immunofluorescence image of the testis tip. Fluorescent in situ hybridization (FISH) to detect expression of the JAK-STAT ligand upd marks the hub (red). Antibody staining against Vasa (green) marks the germ cells. Eight GSCs, indicated by white dots, form a rosette around the apical hub (Adapted from Toledano et al. 2012)
The architecture and function of the testis stem cell niche are influenced by spatially restricted production and secretion of the JAK-STAT ligand Unpaired (Upd), exclusively by hub cells (Fig. 4.1) (Harrison et al. 1998; Kiger et al. 2001; Tulina and Matunis 2001). JAK-STAT signaling acts intrinsically within CySCs to regulate CySC self-renewal and maintenance. In addition, activation of Stat92E, the single Stat orthologue in Drosophila, in CySCs is also important for regulating the behavior of adjacent GSCs in a non-autonomous manner (Flaherty et al. 2010; Leatherman and Dinardo 2008, 2010; Lim and Fuller 2012). Activation of Stat92E within GSCs, however, appears to be important for regulating hub cell-GSC adhesion, rather than proliferation (Leatherman and Dinardo 2010). In addition to the JAK-STAT pathway, Hh (Amoyel et al. 2013; Michel et al. 2012; Zhang et al. 2013) and BMP (Kawase et al. 2004; Leatherman and Dinardo 2010; Michel et al. 2011; Shivdasani and Ingham 2003; Zheng et al. 2011) signaling also play important roles in regulating stem cell behavior within the testis stem cell niche. Therefore, successful spermatogenesis requires adequate signaling between hub cells, CySCs, and GSCs to coordinate proper function of each cell population.
In addition to GSCs that self-renew to maintain the stem cell pool, replacement of lost GSCs can also occur by symmetric GSC divisions, as observed by live cell imaging and lineage tracing (Salzmann et al. 2013; Sheng and Matunis 2011). Furthermore, spermatogonia can dedifferentiate to reacquire stem cell properties to contribute to tissue maintenance (Brawley and Matunis 2004; Sheng et al. 2009). Thus, germline maintenance relies on an intricate balance of renewal/differentiation/dedifferentiation of germ cells that is guided by the niche. This regeneration strategy in place in the testis is highly effective in young adults, but it gradually declines over time.
4.4 Intrinsic Age-Related Changes to Male GSCs
All aspects of spermatogenesis, including stem cell self-renewal, germline differentiation, and gamete maturation, appear to be affected by aging (Boyle et al. 2007; Cheng et al. 2008; Toledano et al. 2012; Wallenfang et al. 2006). Quantification of GSC number in testes of aged (30–35- and 50-day-old) males revealed a significant decrease in the average number of GSCs over time (Fig. 4.2), which appear to be lost as a consequence of detachment from the hub, followed by differentiation and/or cell death (Boyle et al. 2007; Wallenfang et al. 2006). The remaining GSCs progress through the cell cycle more slowly; therefore, both GSC loss and decreased cell division appear to contribute to the decrease in spermatogenesis observed in older males (Boyle et al. 2007; Wallenfang et al. 2006). Phase contrast analysis of testes from older males also shows increased defects in mitotic amplification divisions, although the mechanisms underlying this aspect of germline aging have not been investigated in depth (Boyle et al. 2007).
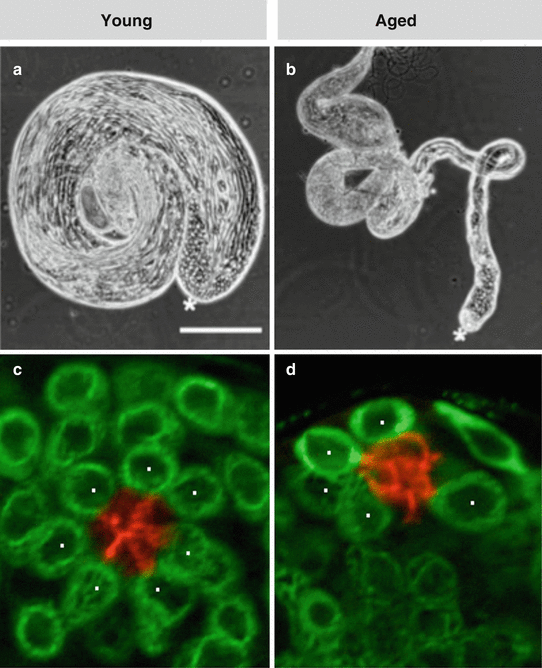

Fig. 4.2
Aging of the Drosophila testis. (a, b) Phase contrast images (in the same magnification, scale bar 250 μm) of testes dissected from (a) a young male (1 day old) or (b) an aged male (50 days old). Note the dramatic reduction in testis size. Asterisks mark apical tip of the testis. (c, d) Immunofluorescence images of testis tips from (c) a young or (d) aged male stained for Fas3 (red, hub) and Vasa (green, germ cells). Note the reduced number of GSCs (indicated by white dots) (Adapted from Boyle et al. 2007)
Using clonal marking strategies, it was demonstrated that GSCs have an approximate half-life of 14 days (Wallenfang et al. 2006). Therefore, one would predict that, given a starting pool of approximately 10 GSCs, testes should be devoid of germline stem cells by 7 weeks; however, testes of 50-day-old males contained an average of five GSCs/testes (Boyle et al. 2007) (Fig. 4.2). This observation indicated that a mechanism must be in place to maintain a minimum stem cell pool. One intriguing observation that was made by using clonal analysis was that the pool of GSCs appeared to progress toward clonality over time (Wallenfang et al. 2006). Therefore, either a few stem cells are more robust and maintained throughout the lifetime of the fly or, alternatively, dedifferentiating spermatogonia could aid in repopulation of the niche. Interestingly, studies have indicated that spermatogonial dedifferentiation does not decline with age and, therefore, could be a major mechanism supporting GSC maintenance during aging (Wong and Jones 2012).
Some insights were provided by studies examining centrosome positioning in male GSCs (Cheng et al. 2008; Yamashita et al. 2003). As mentioned above, Drosophila male GSCs typically divide in an asymmetric fashion with respect to the hub, with the daughter cell destined to differentiate being displaced away from the hub. During interphase, the mitotic spindle is oriented perpendicularly to the hub to facilitate this asymmetric outcome, and centrosome positioning regulates this stereotypical orientation of the mitotic spindle (Yamashita et al. 2003). The mother centrosome in the GSC is located adjacent to the hub, whereas the daughter centrosome migrates to the opposite side (Yamashita et al. 2007). In young adults, GSCs remain oriented perpendicularly to the hub throughout the cell cycle. However, as flies age, the percentage of GSCs with misoriented centrosomes, in which neither centrosome is located at the GSC-hub interface, increases to about 40 % of total GSCs in 30-day-old flies (Cheng et al. 2008). The age-related accumulation in GSCs bearing misoriented centrosomes was attributed primarily to the dedifferentiation process, as mitotic spindles in mitotically dividing spermatogonia are orientated randomly (Cheng et al. 2008). GSCs with misoriented centrosomes do not enter mitosis, but they can restart the cell division once the centrosomes restore their perpendicular positioning (Cheng et al. 2008).
The extra time required for reorientation of the mitotic spindle could be one contributing factor to the decline in the mitotic index observed in GSCs of aged males (Boyle et al. 2007; Wallenfang et al. 2006). In addition, studies have demonstrated that the level of cell cycle regulatory factors is altered in male germ cells (Boyle et al. 2007; Inaba et al. 2011). For example, the levels of cyclin E, a regulator of progression through the G1 phase of the cell cycle, were increased in GSCs of aged males, consistent with an arrest or extension of G1 (Boyle et al. 2007). In addition, String (Stg), the Drosophila homologue of the dual-specificity phosphatase Cdc25, which promotes cell cycle progression, is highly expressed in both germline and cyst stem cells and is rapidly downregulated in differentiating progeny. However, with increasing age, Stg expression declines specifically in GSCs, but not in the somatic CySCs. Loss and gain of function analysis showed that Stg is required for stem cell maintenance and division, and constitutive expression of Stg in the germline rescued the age-related decrease in GSC division and CySC number (Inaba et al. 2011). Thus, further investigation into the age-related decline in Stg expression might lead to additional insights into mechanisms regulating aging of the male germline.
Interestingly, a recent report noted that one of the core histones, histone H3, is asymmetrically segregated during male GSC divisions (Tran et al. 2012). With every cell cycle, newly synthesized H3 is segregated to the daughter gonialblast, while the original pool of H3, presumably carrying the appropriate posttranslational modifications, is maintained in the GSC. If increasing age were to alter the fidelity of H3 segregation, this could be an epigenetic mechanism leading to changes in gene expression and germline differentiation.
4.5 Aging of the Male Germline Stem Cell Niche
4.5.1 Somatic Cyst Cells
Communication between germ cells and somatic cyst cells is essential for regulating GSC proliferation and spermatogonial differentiation and dedifferentiation (Kiger et al. 2000; Leatherman and Dinardo 2008; Matunis et al. 1997; Sheng et al. 2009; Tran et al. 2000). Therefore, changes to CySC behavior over time could contribute to altered germ cell behavior in a non-cell autonomous manner. Staining for Zfh-1, a transcription factor expressed in cyst cells, including CySCs, revealed a significant decrease in the average number of early cyst cells in aged males, similar to what was observed for GSCs (Wong and Jones 2012). However, despite an age-related decrease in early cyst cell numbers, those cyst cells that remain are more active as revealed by an increased percentage of early somatic cells that are progressing through S-phase (Wong and Jones 2012). The increase in cyst cell activity is reminiscent of the behavior of somatic cells in the complete absence of germ cells, as in the case of agametic animals (Gönczy and DiNardo 1996), which may reflect a decline in antiproliferative signals normally emanating from germ cells. Alternatively, the increase in early cyst cell activity with age may represent a mechanism by remaining cyst cells to compensate for the age-related loss of somatic cells.
4.5.2 The Apical Hub
The apical hub cells are specified early in embryogenesis (Boyle and DiNardo 1995), and later during adulthood, hub cells appear to be nondividing and relatively stable with respect to gene expression (Gönczy and DiNardo 1996; Voog et al. 2008, 2014). However, the ability of hub cells to support stem cell behavior changes dramatically with age (Boyle et al. 2007; Toledano et al. 2012). Over time, the 10–12 hub cells appear to become disorganized, and a consistent decrease in the expression of the Drosophila homologue of E-cadherin (DE-cadherin) has been observed (Boyle et al. 2007). DE-cadherin is expressed highly at hub cell-hub cell and hub cell-GSC junctions and is required for maintenance of both male and female GSCs (Inaba et al. 2010; Song and Xie 2002; Song et al. 2002; Voog et al. 2008; Yamashita et al. 2003).
Remarkably, however, roughly 40 % of testes from aged (50-day-old) males showed no detectable expression of a key self-renewal signal, upd. As activation of the JAK-STAT pathways regulates the behavior of both CySCs and GSCs, the decreased expression of upd could contribute to loss of CySCs and/or decreased cell-cell adhesion between GSCs and hub cells directly, as well as decreased GSC proliferation indirectly, by way of altered STAT activity in CySCs. Constitutive re-expression of upd in hub cells suppressed the loss of GSCs of aged males, but had no effect in young males, indicating that loss of upd expression could be a major contributing factor to the age-related decline in spermatogenesis (Boyle et al. 2007). The effects of aging on Hh signaling or the BMP pathway in the Drosophila testis has not been analyzed to date. Interestingly, studies have suggested that decreased expression of self-renewal factors in the murine testis might also contribute to decreased spermatogenesis in this system (Oatley and Brinster 2012; Ryu et al. 2006; Zhang et al. 2006).
The decrease in upd expression in hub cells appears to be due, in part, to the activities of both RNA-binding proteins (RBPs) and small RNAs (both microRNAs (miRNAs) and endogenous small interfering RNAs (endo siRNAs)). The evolutionarily conserved RBP, Imp, possesses K homology (KH) domains that bind the 3′ untranslated region (3′UTR) of upd to stabilize the mRNA. In hub cells, Imp appears to counteract endo-siRNAs that target upd mRNA and, thus, contributes to the maintenance of niche function in young adults (Toledano et al. 2012). However, in testes of older males, the levels of Imp were significantly reduced specifically in hub cells (Toledano et al. 2012). The age-related decrease in Imp also appears to be due to the activity of a small RNA, in this case the conserved heterochronic miRNA let–7, which targets Imp mRNA (Ambros 2011; Toledano et al. 2012). Expression of let–7 is faintly detected in testes from young males; however, in older males, let–7 expression is strongly induced in hub cells
(Table 4.1; Toledano et al. 2012). An age-related increase in let–7 in hub cells leads to a decrease in Imp, which in turn exposes upd mRNA to targeting by endo-siRNAs. Therefore, elevated levels of the heterochronic miRNA let–7 in the hub cells of aged males trigger a cascade of posttranscriptional events that result in decreased JAK-STAT signaling and impaired niche function over time (Toledano et al. 2012).
Table 4.1
Deep sequencing data of Drosophila melanogaster (dme)-miRNAs in libraries from testis of wild-type (OregonR) young (1 day) and aged (30 day) old flies
miRNA | Sequence | 1 day adult testis library | 30 day adult testis library | Ratio 30 day/1 day | ||
|---|---|---|---|---|---|---|
No. of reads | % of lib. | No. of reads | % of lib. | |||
dme-let-7-5p | TGAGGTAGTAGGTTGTATAGT | 1,17,568 | 0.98 | 2,17,661 | 1.86 | 1.90 |
dme-mir-100-5p | AACCCGTAAATCCGAACTTGT | 1057 | 0.01 | 2049 | 0.02 | 1.99 |
dme-mir-125-5p | TCCCTGAGACCCTAACTTGTGA | 2534 | 0.02 | 5053 | 0.04 | 2.05 |
Notably, constitutive expression of Upd or Imp that is not susceptible to let-7-mediated degradation in hub cells suppresses GSC loss in older males (Boyle et al. 2007; Toledano et al. 2012). Nonetheless, forced Upd/Imp expression was not sufficient to fully rescue defects in spermatogonial proliferation and differentiation, confirming that aging independently modulates other stages of spermatogenesis.
4.5.3 Systemic Signals
Numerous studies have indicated that stem cells within a variety of tissues and species are influenced by insulin/IGF signaling (Shim et al. 2013). The insulin signaling pathway, which is conserved in Drosophila, encodes a single insulin/IGF-like receptor (dInR) and seven insulin-like peptides (dILPs) in its genome (Brogiolo et al. 2001; Ikeya et al. 2002; Puig et al. 2003). Experiments have clearly demonstrated that insulin signaling directly regulates the rate of GSC proliferation in the Drosophila ovary (Ikeya et al. 2002; LaFever and Drummond-Barbosa 2005; Yu et al. 2009) and regulates the maintenance of female GSCs indirectly by preservation of the niche (Hsu and Drummond-Barbosa 2009). Clonal analysis demonstrated that dInR function is also required cell autonomously for GSC maintenance in the Drosophila testis, suggesting that male GSCs are competent to receive and respond to insulin signaling directly (McLeod et al. 2010; Ueishi et al. 2009). Importantly, dInR appears to regulate GSC maintenance independently of the JAK-STAT pathway (McLeod et al. 2010; Wang et al. 2011).
Three dILPs, dILP2, dILP3, and dILP5, are expressed in insulin-producing cells (IPCs) in the brain, secreted into the circulating hemolymph, and act to regulate InR activity systemically (Brogiolo et al. 2001; Ikeya et al. 2002; Kramer et al. 2003; Puig et al. 2003). While a significant source of dILPs is in the brain, expression of dILP5 and dILP7 has been reported in the ovary and female reproductive tract, where they regulate the onset of vitellogenesis and coordinate the strong and swift response of the female reproductive program to nutrition (Slaidina et al. 2009; Yang et al. 2008). In contrast, dILP2 and dILP3 are expressed in somatic cyst cells in the testis that encapsulate spermatocytes and differentiating cysts at the basal end of the testis (McLeod et al. 2010




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree