CHAPTER 2 Adverse Sequelae and Complications of Venous Hypertension
Pathogenesis
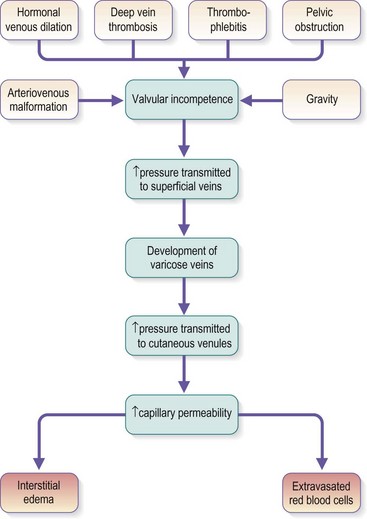
Chronic venous insufficiency, which must be semantically distinguished from venous disease or disorder, may be defined as relative impedance of venous flow back to the heart and responsible for clinical consequences. When this occurs in the lower extremities, the normal reabsorption of perivascular fluids by osmotic and pressure gradients is impaired, resulting in accumulation of perivascular and lymphatic fluid. This leads to edema and impaired oxygenation of surrounding tissue (Figs 2.1 and 2.2). This disruption of the normal vascular and lymphatic flow of the lower extremities may result in pain, cramping (especially at night), restlessness, pigmentary changes, dermatitis, and ulceration (Fig. 2.3).1–3 The association of abnormal venous flow with various signs and symptoms has been noted for centuries: first by Hippocrates in the fourth century BC and by Wiseman in England in 1676.4 It has been estimated that chronic venous insufficiency will develop in almost 50% of patients with major varicose veins.5,6
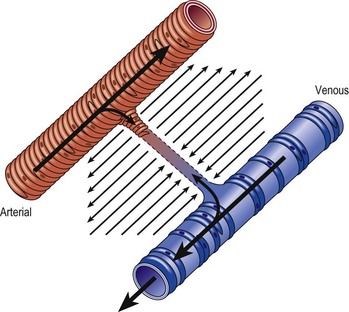
Several alterations of normal venous flow cause venous hypertension. Such hypertension in the lower extremities is usually caused by a loss or disruption of the normal one-way valvular system. This may occur because of deep vein thrombosis (DVT), thrombophlebitis, or a dilation of veins from other causes.7,8 When perforating vein valvular function becomes incompetent, there may be shunting of blood flow from the deep to the superficial venous system through the incompetent perforating veins, with resultant adverse sequelae.9–11 The superficial veins respond by dilating to accommodate the increased blood flow, which produces superficial valvular incompetence leading to the development of varicosities.12 In addition, with muscular movement in the lower limbs, the high venous pressure normally occurring within the calf is transmitted straight to the superficial veins and subcutaneous tissues.13,14 Venous pressure in the cuticular venules may greatly exceed the normal 100 mmHg in the erect position.15 This causes venular dilation over the whole area, resulting in capillary dilation, increased permeability,16–19 and increase in the subcutaneous capillary bed.17,20 This is manifested as telangiectasia and venectasia (Fig. 2.4). Venous hypertension has also been demonstrated to destroy the venous valves that are present in the subcuticular vascular system.21 This destruction promotes persistent and progressive changes in the venous drainage system of the skin and subcutaneous tissues. The greater the degree of venous hypertension, the greater the risk of venous ulcer development.22,23 Fortunately, both sclerotherapy and surgical treatment are capable of normalizing abnormal venous hypertension.
Venous hypertension and subsequent insufficiency may also derive from venous obstruction, either at lower limb level or at iliocaval level, usually associated with reflux.24
Molecular Mechanisms
The molecular mechanisms involved in leukocyte adhesion and activation in chronic venous disease (CVD) are beginning to be elucidated. Circulating leukocytes and vascular endothelial cells express membrane adhesion molecules. For example, binding of L-selectin on the leukocyte surface to E-selectin on endothelial cells may be involved in leukocyte ‘rolling’ along the endothelial surface (Fig. 2.5A). Then, when leukocytes are activated, they shed L-selectin into the plasma and express molecules of the integrin family, including CD11b, which binds firmly to intercellular adhesion molecule-1 (ICAM-1). Integrin binding can promote firm adhesion of leukocytes, the starting point for their degranulation8 and migration through the endothelium.
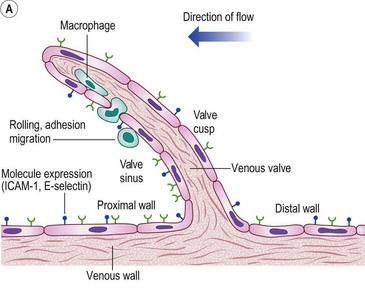
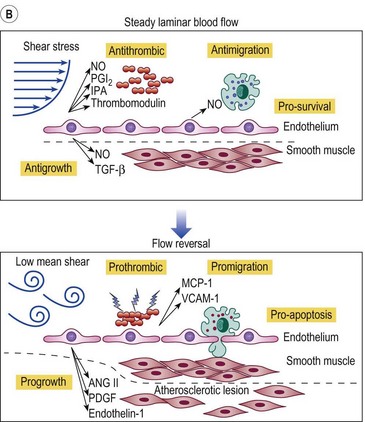
Figure 2.5 A: Leukocyte-endothelial interactions on a venous valve. ICAM-1, intercellular adhesion molecule-1. B: Summary of contrasting effects of steady, laminar shear stress (upper panel) and turbulent or reversing shear stress (lower panel) on vessel walls. NO, nitric oxide; PGI2, prostacyclin; t-PA, tissue plasminogen activator; TGF-β, transforming growth factor beta; MCP-1, monocyte chemotactic protein-1; VCAM-1, vascular cell adhesion molecule-1; ANG II, angiotensin II; PDGF, platelet-derived growth factor.
(Modified from Traub O, Berk BC: Arterioscler Thromb Vasc Biol 18:677, 1998, with permission.)
(Modified from Takase S, Bergan JJ, Schmid-Schönbein GW: Ann Vasc Surg 14:427, 2000 and from Coleridge Smith PD, Bergan JJ: Inflammation in venous disease: In Schmid-Schönbein GW, Granger N, editors, Molecular basis for microcirculatory disorders, Paris, 2003, Springer-Verlag, pp 489–500, with permission.)
Several studies have looked at markers of leukocyte and endothelial adhesion and activation in CVD. After venous hypertension was induced by standing for 30 minutes, levels of L-selectin and integrin CD11b on circulating neutrophils and monocytes in CVD patients were found to decrease, reflecting the trapping of these cells in the microcirculation. At the same time, plasma levels of soluble L-selectin increased, reflecting the shedding of these molecules from leukocyte surfaces during leukocyte–endothelial adhesion.24 Similarly, basal plasma levels of the adhesion molecules ICAM-1, endothelial leukocyte adhesion molecule-1 (ELAM-1), and vascular cell adhesion molecule-1 (VCAM-1) were higher in CVD patients than controls and increased significantly in response to venous hypertension provoked by standing.25 Baseline levels of plasma von Willebrand factor, a marker for endothelial cell damage, were also higher in patients with lipodermatosclerosis than in those without skin changes.26 Lactoferrin and neutrophil elastase are enzymes released from neutrophil granules. Plasma levels of these molecules are therefore markers of neutrophil activation, and both were found to be higher in patients with CVD than in age- and sex-matched controls.27
In most of these studies, prolonged standing induced venous hypertension. However, in patients with chronic venous insufficiency, increases in plasma levels of ICAM-1 and VCAM-1 can be induced by walking,28 presumably in response to hydrodynamic pressure increases generated by the musculovenous pump being transmitted to subcutaneous and cutaneous small vessels through incompetent perforating veins.
In addition to local factors operating in relation to venous hypertension, CVD patients have a tendency for systemically elevated leukocyte adhesion. Venous hypertension in the upper limb (where the local vessels and tissues are presumably normal) produced by means of a pressure cuff causes greater leukocyte accumulation in patients with venous leg ulcers than in normal subjects.29 Evidence has been found for an activation factor in the plasma of CVD patients.15 Plasma obtained from CVD patients induced greater degrees of activation as shown by oxygen-free radical production and pseudopod formation in healthy, naive granulocytes than did plasma taken from normal subjects. Also, there was a trend towards more activation in more severe cases of chronic venous insufficiency. The nature of the plasma factor responsible for leukocyte adhesion and activation is presently unknown.
Inflammation and Skin Changes
There has also been progress in linking the chronic inflammatory state seen in CVD patients with the specific skin changes typical of the condition. In lipodermatosclerosis, the skin capillaries become elongated and tortuous, giving the appearance in histologic sections of elevated capillary density.30 In advanced skin disease especially in the ulcerative stages, the capillaries may take on a glomerular appearance,31 and it seems clear that substantial proliferation of the capillary endothelium occurs. Several factors could contribute to endothelial proliferation, but vascular endothelial growth factor (VEGF) is an obvious candidate. VEGF is known to be involved in inflammatory and healing processes in the skin and has been shown to increase microvascular permeability both acutely and chronically.32 Plasma levels of VEGF have been shown to increase during the venous hypertension induced by 30 minutes of standing both in normal subjects and in CVD patients. Both supine and standing VEGF levels are higher in patients than in normal controls.33 Furthermore, plasma VEGF levels are higher in CVD patients with skin changes than in CVD patients with normal skin.34
Another feature of the skin changes associated with CVD is dermal tissue fibrosis. Transforming growth factor-β1 (TGF-β1) is a known fibrogenic cytokine. Detailed analysis of punch biopsy specimens has shown that skin from the lower calf of CVD patients had significantly elevated active TGF-β1 levels compared with normal skin. In addition, this was true relative to skin taken from the lower thigh region of the same patients.35 Immunohistochemistry and immunogold labeling showed the TGF-β1 to be located in leukocytes, fibroblasts, and on collagen fibrils. Pappas el al36 proposed that activated leukocytes migrate out of the vasculature and release TGF-β1, which stimulates increased collagen production by dermal fibroblasts. Over an extended period, such a process could contribute to the typical dermal fibrosis seen in CVD.
Altered collagen synthesis has also been reported for dermal fibroblasts taken from apparently healthy areas of skin in patients with varicose veins.37 It has been possible to correlate altered levels and distributions of growth factors, including basic fibroblast growth factor (bFGF), transforming growth factor-3 (TGF-3), and the receptor for epidermal growth factor (EGF), with different types of skin change, including venous eczema, pigmentation, lipodermatosclerosis, and ulceration.38
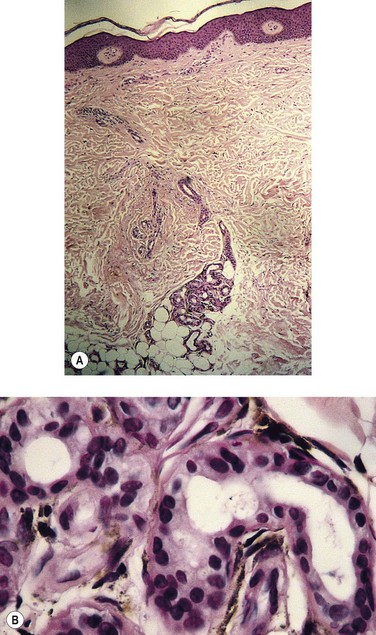
Advanced cutaneous changes of lipodermatosclerosis appear as an erythematous, telangiectatic, edematous plaque with mottled hyperpigmentation. Histologic examination shows advanced stasis changes, including zones of ischemic necrosis in the central part of fat lobules. Septal fibrosis, fat microcysts, membranous fat necrosis, and sclerosis occur later. This advanced clinical–histologic change is termed sclerosing panniculitis.39 With superficial venous insufficiency alone, changes in the histologic appearance of capillaries are moderate. The combination of deep venous insufficiency, with or without superficial venous insufficiency, produces more profound changes.40,41
Labropoulos et al42 studied 255 limbs in 217 patients. These had superficial venous insufficiency alone, with normal perforating veins and deep veins. The color-flow duplex imaging techniques were used. The researchers concluded that aching, ankle edema and skin changes in limbs with reflux confined to the superficial venous system were associated predominantly with reflux in veins below the knee. An important finding was that ulceration occurred only when the entire great saphenous vein (GSV) was involved or when reflux was extensive in both the great and small saphenous systems.
Classification of Venous Disease
An international ad hoc committee of the American Venous Forum developed the CEAP classification for CVD in 1994. The goal was to stratify clinical levels of venous insufficiency. The four categories selected for classification were: clinical state (C), etiology (E), anatomy (A), and pathophysiology (P). The CEAP classification has been endorsed worldwide despite its acknowledged deficiencies. It has been adopted as a standard in many clinics in Europe, Asia and South America and is considered the only modern method for reporting data in the United States.43–45
The CEAP classes are shown in Box 2.1. Each clinical class is further characterized by a subscript for the presence of symptoms (S, symptomatic) or their absence (A, asymptomatic). Symptoms include aching, pain, tightness, skin irritation, heaviness, and muscle cramps, as well as other complaints attributable to venous dysfunction. A basic CEAP is suggested. For the practicing physician, CEAP is an instrument for correct diagnosis, to guide the treatment and assess the prognosis. Venous disease is complex, but can be described. In modern phlebologic practice, the vast majority of patients will undergo a duplex scan of the venous system of the leg, which will provide data on E, A and P. In basic CEAP where a duplex scan is performed, E, A and P should be utilized. For the anatomical classification A in basic CEAP, the simple s = superficial, p = perforator and d = deep descriptors should be used. Multiple descriptors should be permitted for all four components in basic CEAP; for example a patient could be classified as C234s Ep Asd Pr. Use of all components of CEAP is encouraged. Definitions that apply to the CEAP classification are shown in Box 2.2.
Box 2.2 CEAP definitions
But the CEAP is a description of the disease, not an assessment of its severity. It serves for classification, not evaluation. For this reason, several scores have been added to the CEAP, such as the VCSS (venous clinical severity score).46 However, a physician reported outcome such as the VCSS is influenced by the ‘experimenter-expectancy effect’ and so the need for the application of patient reported outcomes (PROs) to clinical trials is now recognized.47 Revicki summarizes: ‘… the patient’s perspective and patient-reported HRQL (health related quality of life) is the ultimate outcome for health care interventions.’48 Basically, a PRO will increase the validity of randomized controlled trials (RCTs) especially when they cannot be double or even single blinded. The influence of the experimenter’s opinion about the efficacy of a treatment significantly changes the RCT results in many different fields of medicine.49 An additional precaution for outcome evaluation could be the use of an external evaluation, but this would not take into account the patient’s point of view and self-appraisal.
The patient’s opinion is a point that, albeit obvious, has been too long neglected. Several Quality of Life questionnaires, generic (e.g. SF1250) and specific (e.g. CIVIQ,51 AVVQ) already exist and have been successfully used.52 However, they have not take into account the only sure thing we could ever state: if the patient is not happy with the result of the treatment, it means it (you) failed. Therefore, the need to assess the patient’s own appraisal in detail, with satisfactory sensitivity and specificity, appears of utmost importance. Guex et al addressed this point and observed that CVD patients had one main concern belonging to one of the following five groups: discomfort/pain, appearance/attractiveness, risk/threat to health, restriction of movements/activities, emotional distress.53 They have therefore constructed a novel PRO (the SQOR-V), specially dedicated to CVD and based on these five patient concerns.54 Its values range from 20 to 100, 20–30 being normal, 30–50 moderate consequences of CVD and >50 being severe CVD. This questionnaire is free to use and is available in several languages, including French, English and Spanish.
Pittaluga et al have established a classification of venous refluxes based on the extent of saphenous vein reflux.55 They noted a positive correlation between age and progression of superficial venous insufficiency.
Incidence
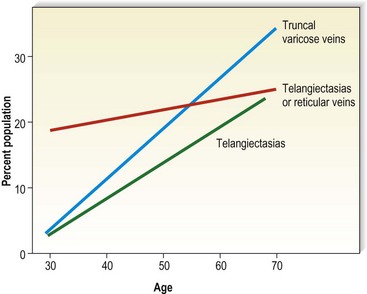
Varicose veins have been noted to increase in incidence with age and have been estimated to occur in 7% to 60% of the adult population in the United States.5,15,56–60 Varicose veins occur in 8% of women aged of 20 to 29 years, increasing to 41% in the fifth decade and 72% in the seventh decade of life.57,61,62 A similar rate of increase in the incidence of varicose veins occurs in men: 1% in the third decade, increasing to 24% in the fourth decade and 43% in the seventh decade.57,61 The Basle study III found that the greatest correlation between age and incidence of varicose veins occurred in those with varicose veins only, rather than in those with the presence of telangiectasias (hyphenwebs) or reticular veins (Fig. 2.6).5 The Framingham study,63 curiously, showed no difference in the incidence of varicose veins with age.
Varicosities in childhood are rare, occurring almost exclusively in association with congenital vascular malformations (see Chapter 3). When varicosities occur, they usually appear as a subtle physical finding, such as a slight bulge over the popliteal fossa (Fig. 2.7). One estimation on the incidence of telangiectasias in children was performed by Oster and Nielsen64 in Denmark. Of 2171 Danish schoolchildren examined, 46.2% of the girls and 35.1% of the boys had telangiectasias on the nape of the neck; 1% of these children had pronounced telangiectasias elsewhere; that is, on the shoulders, thorax, cheeks and ears. No mention was made of the occurrence of telangiectasias on the legs. An examination of 403 children in the former East Germany, aged 8–18 years, disclosed a 50% incidence of ‘very discrete’ venous abnormalities of leg veins. Of the children examined, 15% had clear symptoms (without visible varices) that could be assigned to a prospective varicose disease. Only between the ages of 17 and 18 years could reticular varicose veins be identified. In the 403 children studied by venous Doppler, 2.3% had incompetent communicating veins and 3.2% had an incompetent saphenofemoral junction.65 An epidemiologic study of 419 Czechoslovakian children aged 8 to 13 years, with clinical examination plus digital photoplethysmography, showed clinically apparent varices in 8.7% and an impairment of venous function in 14.3% of the examined extremities.66
The most recent and complete studies of 518 children aged 10 to 20 years, using photoplethysmography in addition to venous Doppler, are the Bochum I, II, and III studies. This continuing investigation initially demonstrated a 10.2% incidence of reticular varicose veins without other venous abnormalities in children who were 10 to 12 years of age.67 When these children were examined 4 years later, the incidence of reticular veins was 30.3%; 2% of the children had developed varicose veins and 4% had developed incompetent communicating veins. When they were examined another 4 years later, the frequency of saphenous refluxes and large varices was still increasing (19.8% saphenous refluxes, 3.3% truncal varices, 5% tributary varices, and 5.2% incompetent perforators). The incidence of reticular veins increased to 35.5%, and the incidence of telangiectasia increased to 12.9%.68 Therefore, venous disease can be demonstrated in a small number of children, its progression can be documented, and saphenous reflux is a risk factor for the development of truncal varices.
Symptoms
Varicose veins may be symptomatic in addition to being large and unsightly (Box 2.3). A study of 4280 town and country inhabitants of Tübingen, Germany, found symptoms in 98% of patients with clinically relevant venous alterations.69 One American health survey found that nearly 50% of those with varicose veins were bothered by their symptoms once in a while, and 18% noted frequent to continuous symptoms.70 Galen71 described the symptoms of varicose veins as ‘a heavy and depressing pain’. Pain is related most likely to pressure on the dense network of somatic nerve fibers present in the subcutaneous tissues adjacent to the affected nerve. Alternatively (or in addition), pain may occur from the dilated vein compressing adjacent nerves or from lactic acid accumulation that results from retrograde, circular, or slower venous blood flow clearance. Symptoms may precede the clinical appearance of the varicosity and are proportional to the presence of intermittent edema. At this point, discomfort usually occurs during warm temperatures when the patient is standing for prolonged periods.72 These patients may respond to systemic hydroxyrutosides, which decrease inflammation of the vein wall.73–75 Paroven – a mixture that mainly consists of mono-, di-, tri-, and tetra-O-β-hydroxyethyl rutosides containing at least 45% troxerutin (Zyma, United Kingdom) – 250 mg three or four times a day has been reported to help.76 Reduction in venous hypertension or excision of the involved segment usually causes prompt relief of pain.4
Almost all patients with postthrombotic obstruction complain of ‘bursting’ calf pain that is exacerbated by exercise.77 Venous claudication is an appropriate term to use in this situation to emphasize the relationship of exercise to pain. Pain during exercise may be due to nociceptor stimulation of the distended vein wall.78 Alternatively, exercise pain may be due to an accumulation of tissue metabolites or an increase in interstitial pressure. Patients with acute DVT have an increased intramuscular pressure that is proportional to the degree of thrombosis.79,80
Kistner81 has found that perforator incompetence leads to indurated skin with ulceration, whereas incompetence of the tibial, popliteal, or femoral system produces aching and swelling in the leg. There are patients who have a significant amount of valvular incompetence without symptoms. Clinical symptoms vary based on the effectiveness of the calf muscle pump to compensate for venous hypertension. Young, thin, and more athletic patients have fewer symptoms than older and obese patients.
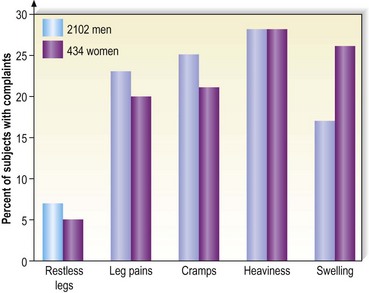
Varicose vein pain is described usually as a dull aching of the legs, particularly after prolonged standing or during certain times of the menstrual cycle (especially during menses).82 A small number of women also experience painful varicose veins after sexual intercourse.83 It is proposed that the increase in venous pressure with distension of the varicose vein contributes to heaviness and tightness of the lower legs.4,84 The Basel study III found a similar range of complaints in both men and women (Fig. 2.8) and an increased incidence of complaints among women and older patients.5 Many patients in the Basel study III had complaints without evidence of peripheral vascular disease. Strandness and Thiele4 suggest that symptoms specifically caused by varicose veins improve with aging as the level of activity declines.
Symptoms in varicose veins are often disproportionate to the amount of actual pathologic change. Patients with small, early-stage varices may complain more than those with large, longstanding varicosities.85–89 A survey of 350 patients who presented for sclerotherapy treatment of veins that were less than 1 mm in diameter noted that 53% of these patients complained of swelling, burning, throbbing and cramping of the legs, in addition to a ‘tired feeling’.90 A retrospective analysis of 401 patients who sought treatment solely for telangiectasia showed 69% with various symptoms, including pain, cramping, burning, throbbing and heaviness.91 These symptoms are so insidious that most patients fail to realize how good their legs can feel until after treatment of these blood vessels by either compression sclerotherapy or by wearing lightweight (20 mmHg) graduated compression stockings.59 Patients with nonsaphenous reflux had twice the incidence of painful legs (43%) than did patients with saphenous reflux (22%).92
The increased incidence of symptomatic varicose veins in women may have a hormonal etiology. It has been estimated that 27.7% of women with varicose veins have premenstrual pain in their varices.93 Varicose veins during pregnancy appear to be more symptomatic than those unassociated with pregnancy. In a study of 150 pregnant women with varicose veins, 125 noted pain and 26 were unable to stand for more than 1 to 2 hours because of the pain.94
The differential diagnosis of leg pain is extensive and not necessarily attributable to a patient’s varicosities (Box 2.4). Symptoms derived from varicose veins can usually be distinguished from arterial symptoms. Pain associated with arterial diseases often disappears at rest and is exacerbated with walking. Pain associated with varicosities is dull, vague and localized on the medial side of the legs. It is usually relieved with walking. In addition to the dull aching, varicose veins associated with venous hypertension may produce cramping or painful spasms of the legs and an increase in leg fatigue and restless legs, especially at night. Unfortunately, a patient’s perception and reporting of pain are subjective in nature and difficult to document. Browse et al95 advocate the use of compression hosiery as a diagnostic test to determine if the pain is of venous origin. The authors have found that if a patient’s symptoms are alleviated with 20 mmHg graduated compression stockings, sclerotherapy treatment also alleviates the symptoms.
In a study that compared groups matched by age as well as gender, it was found that subjects with venous disease were markedly symptomatic compared with control individuals. Symptoms specific to venous disease were found to correlate with the presence of both small vein and large vein disease. Vein size did not predict the presence or severity of symptoms.96 A study of 1366 people in Edinburgh, Scotland, found that women were more likely than men to report a wide range of lower limb symptoms.97 In men, only itching was significantly related to the presence of varicose veins. In women, there was a significant relation between varicose veins and heaviness or tension, aching and itching.
Using the Aberdeen varicose vein questionnaire, a prospective cohort of 137 patients was studied and the results compared with the well-accepted short form (SF-536). The two questionnaires correlated highly. Both showed the patients having worse health preoperatively than postoperatively. After surgery, all domains of health were improved and reached significance, chiefly in mental health. The conclusion of the authors of this study was that persons with varicose veins have a reduced quality of life (QOL) compared with the general population and that this discrepancy is significantly improved at 6 weeks after surgery.98
Two recent QOL studies were conducted on people with varicose veins. An analysis of 5688 consecutive outpatients in Belgium, Canada, France, and Italy found that the QOL in patients with varicose veins is associated with concomitant venous disease, rather than the presence of varicose veins per se.99 Sixty-five percent of patients with varicose veins also have concomitant venous diseases such as edema, skin changes or ulcers. A study of 2404 employees of the University of California, San Diego Medical Center, also found an adverse effect on QOL in patients with CVD.100 The effect of venous disease is more on the functional scale (what a person can do) and does not seem to affect the well-being aspects (how a person feels). Thus, venous disease is more than simply a cosmetic problem to most patients.
Signs
In addition to the direct symptoms of varicose and telangiectatic veins, varicose veins may be a cutaneous marker for venous insufficiency, where they occur in more than 50% of patients (Box 2.5).101–104 Certainly, deep venous insufficiency as a result of valvular incompetence is the major etiologic factor for the cutaneous manifestations of chronic venous insufficiency. In fact, when descending venography was used to examine patients with chronic venous insufficiency, reflux occurred in the superficial system alone in only 2% of the 644 examined limbs. Eighteen percent of the limbs had combined deep and superficial reflux.105 Studies using ascending venography have shown that more than 20% of patients with chronic venous insufficiency have isolated perforator incompetence as the only demonstrable abnormality.106 However, a significant group of patients has superficial venous insufficiency alone (13–38%) or in combination with deep venous insufficiency (28–78%).104,107–112 In addition, Walsh et al113 found that treating the incompetent superficial venous system with ligation and stripping the GSV to the knee with stab avulsion of distal varices restored competency to the femoral vein. Therefore, identification of patients with superficial venous insufficiency is important because they may respond to sclerotherapy or surgical treatment of the superficial venous system alone.
It has been estimated that between 17% and 50% of the population with varicose veins have cutaneous findings.2,114 Approximately 70% of limbs with chronic venous insufficiency have clinical findings.108 There is a strong association between the severity of clinical signs (described later in this chapter) and superficial venous incompetence.108 Almost all patients with cutaneous abnormalities have incompetence of perforator veins and all patients with either active or healed venous ulcerations have evidence of perforator incompetence.114 The cutaneous manifestation appears as edema, hyperpigmentation, dermatitis or ulceration. More than a million Americans suffer from skin ulcers caused by abnormal circulation that results from varicose veins, and nearly 100,000 Americans are totally disabled by this condition.57 Thus, varicose and telangiectatic leg veins are not merely of cosmetic concern but represent a widespread and potentially serious medical problem.
Edema
Ankle edema is usually the first manifestation of chronic venous insufficiency.115 Ankle edema tends to be worse in warm weather2 and towards the end of the day.59 It is especially common in persons who stand a great deal.59 True ‘pitting’ edema is rare,85 perhaps resulting from increased dermal fibrosis present in lipodermatosclerosis. The edema usually found is restricted to a limited area drained by capillaries that empty directly into the varicose or incompetent perforating veins.116 This area has been termed the gaiter area and refers to the ankle and lower calf. (In the 1800s it was commonly covered by a cloth or leather material (gaiter) to protect the ankle and instep from the environmental elements. Such protection is still used today by cross-country skiers.) Ankle edema caused by venous hypertension and varicose veins must be differentiated from that caused by other conditions (Box 2.6). However, as previously described, lymphatic edema may also be present in patients with chronic venous leg ulcers.117
The incidence of leg edema may not be related to the extent of varicose vein disease. A statistical study of 9100 civil servants in the German cities of Düsseldorf and Essen disclosed a statistically significant increase only in leg swelling in those with leg veins less than 1 mm in diameter compared to in those without such veins.118 There was no difference in muscle cramps, restless legs, and itching. Pain was not evaluated.
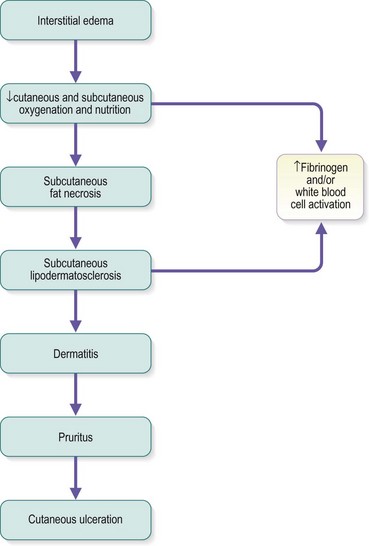
The protein-rich edema fluid stimulates fibroblastic activity, which entangles blood vessels and lymphatics into a fibrous mass.119 Histologically, a microedema around capillaries is seen.120 The edema, which contains fibrin, proteins and neutral polysaccharides, is probably the main reason for the lack of nutrition of the skin.121,122 The resulting lymphedema and the hypertrophy of the skin and subcutaneous tissues disrupt the flow of cutaneous nutrition. In women with venous stasis and fat, hairless, erythrocyanoid-type ankles, the resulting decrease in nutrition for the sizable fatty subcutaneous tissue and the decrease in local tissue oxygenation may cause sudden and massive fat necrosis of the subcutaneous tissue.2,15 The affected area may then appear erythematous, indurated, and tender to the touch.
Guex et al have correlated ankle circumference, symptoms and quality of life scores in 1036 patients with venous symptoms.123 They demonstrated the relevance of moderate ankle swelling to be secondary to chronic venous insufficiency.
Treatment of ankle edema caused by increased venous pressure, with or without lipodermatosclerosis, is directed primarily towards the prevention of trauma and alleviation of superficial venous hypertension.19 Temporizing treatments include leg elevation, systemic diuretics and localized compression bandaging.19 Graduated compression bandaging can normalize lymphatic flow over time. Thus this ‘conservative’ form of treatment is actually therapeutic as well (see Chapter 6). Recently, fibrinolytic enhancement has reportedly been successful in improving symptoms, induration and cutaneous thickening in patients with lipodermatosclerosis.124 The authors of that report used stanozolol (5 mg by mouth, twice daily) in 14 patients with longstanding lipodermatosclerosis resulting from venous disease. Of these 14 patients, 11 noted improvement within 3 months. Long-term follow-up was not given, nor has this treatment been accepted elsewhere.
Pigmentation
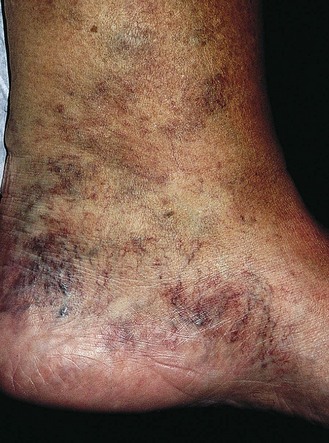
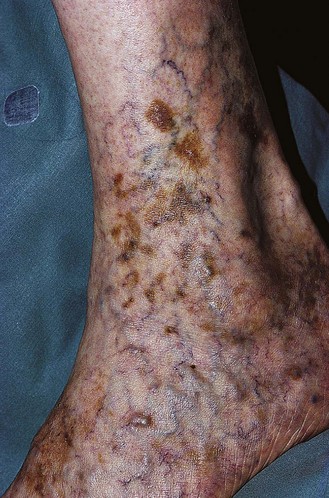
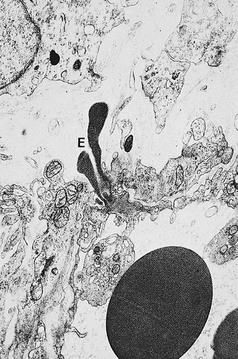
Pigmentation (Fig. 2.9) is a sign of venous stasis disease. The elongated, distended vascular system underlying areas of stasis is more susceptible to trauma than are normal vessels. Even minor blunt injuries may cause rupture of the vascular wall with extravasation of erythrocytes into the cutis.12,18,119 Histologically, cutaneous hyperpigmentation represents increased melanin early on and extravasated erythrocytes and hemosiderin-laden macrophages interspersed between dilated and tortuous capillaries in later stages and in inflammatory stages.12,125,126 Erythrocytes appear either intact or to have become fragmented during their passage (Fig. 2.10). Extravasation appears to be due to increased intravascular pressure and not chemotaxis, as occurs with white blood cells.125
Extravasated erythrocytes may be found in the deep dermis around adnexal structures (Fig 2.11). When present clinically, they are a reliable guide to the existence of microangiopathy. A more acute ‘eruptive’ cutaneous pigmentation has also been noted as a ‘blow-out’ of erythrocytes into the dermis as a result of tremendous back pressure within the cutaneous microvasculature.127 This phenomenon has been ascribed as the cause of lichen aureus (Fig. 2.12).128 The only treatment for this condition is correction of the underlying venous hypertension (sclerotherapy and/or surgery), including graduated compression stockings and leg elevation. Fortunately, when venous hypertension is treated, dark pigmentation gradually fades. After correction of the etiology for the venous hypertension, a Q-switched laser specific for hemosiderin can then be used to remove or minimize the pigmentation (see Chapter 8). In addition, a noncoherent intense pulsed light source has also been demonstrated to lighten pigmentation in one case report.129
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree