Fig. 8.1
A 56-year-old male 5 years status post renal transplant with 0.4 cm nodule on the left zygomatic cheek
MCC is most likely to occur approximately 6–8 years after transplantation, and the incidence dramatically increases in patients who are greater than 20 years post-transplant [36]. 69 % of post-transplant MCCs occur in male patients partly due to the predominance of men among the OTR population. The majority of MCCs in OTRs, up to 93 % of MCC in OTRs, are diagnosed in renal transplant patients, with heart and liver transplant patients each accounting for approximately 4 % [23].
In three reports involving the withdrawal of immunosuppression in OTRs with MCC, the patients remained disease free for approximately 6 months before developing rapidly progressive disease [34, 37, 38]. In contrast, partial to complete spontaneous regression of MCC in patients without iatrogenic immunosuppression, approximately 20 cases, has also been reported in the literature [34, 39]. Most of these cases occurred soon after a biopsy was performed, likely indicating that trauma stimulated the observed T cell–dominant immune response. In these reports, patients remained disease free decades after regression.
8.6 Clinical Features
The clinical features most commonly associated with MCC are summarized by the mnemonic “AEIOU” and can be used to aid in diagnosis. In tumors that have three or more of the following, a diagnosis of MCC should be suspected: Asymptomatic/nontender, Expanding rapidly, Immunosuppressed, Older than 50 years, and extensive UV damage in a fair-skinned patient. Eighty-nine percent of MCC will have three or more of these features [40].
While MCC most frequently appear on sun-exposed areas, most commonly the head/neck (49 %), followed by the extremities (45 %) and the trunk (15 %) (Fig. 8.2), they can also occur on completely sun-protected areas such as the buttock and vulva [41]. OTRs may have multiple MCCs, and most have other types of skin cancer [23, 30]. The average size at diagnosis in OTRs is 2 cm [42].
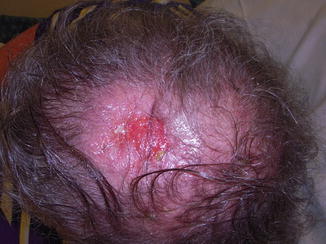

Fig. 8.2
A 66-year-old male 5 years status post bilateral lung transplant with 3.5 cm primary scalp lesion
8.7 Staging and Prognosis
The American Joint Committee on Cancer (AJCC) MCC staging system was first published in 2010 [43] (Table 8.1). The percentage of patients with localized disease at presentation ranges from 49 to 76 %, with most of the cases of localized disease presenting on the extremities [8, 10, 42].
Table 8.1
AJCC staging system
Tumor | Regional nodes | Distant metastasis |
|---|---|---|
TX – primary tumor cannot be assessed | NX – regional lymph nodes cannot be assessed | M0 – no distant metastasis |
T0 – no evidence of primary tumor(e.g., nodal/metastatic presentationwithout associated primary) | N0 – no regional lymph nodes metastasis | M1 – metastases beyondregional lymph nodes |
Tis – in situ primary tumor | cN0 – nodes negative by clinical exam(no pathologic node exam performed) | M1a – metastases to the skin,subcutaneous tissues, or distantlymph nodes |
T1 – ≤2 cm maximum tumordimension | pN0 – nodes negative by pathologic exam | M1b – metastasis to lung |
T2 – >2 cm but ≤5 cm maximumtumor dimension | N1 – metastases in regional lymph node(s) | M1c – metastases to all othervisceral sites |
T3 – >5 cm maximum tumordimension | N1a – micrometastasis | |
T4 – primary tumor invades thebone, muscle, fascia, or cartilage | N1b – macrometastasis | |
N2 – in-transit metastasis |
Stage | T | N | M |
|---|---|---|---|
0 | Tis | N0 | M0 |
IA | T1 | pN0 | M0 |
IB | T1 | cN0 | M0 |
IIA | T2/T3 | pN0 | M0 |
IIB | T2/T3 | cN0 | M0 |
IIC | T4 | N0 | M0 |
IIIA | Any T | N1a | M0 |
IIIB | Any T | N1b/N2 | M0 |
IV | Any T | Any N | M1 |
The relative probability of survival decreases significantly with the finding of regional or metastatic spread. MCC patients in the general population who present with local disease have shown a relative survival of 64 % at 5 years, while those with regional nodal disease have a 5-year relative survival that decreases to 39 % (Fig. 8.3). Patients who present with distant metastatic MCC have a dismal 5-year relative survival of only 18 % [44]. Few patients diagnosed with distant metastases survive for 3 years or longer [45]. In OTRs, MCC is even more aggressive than in the general population with 68 % of OTRs presenting with lymph node involvement and 56 % dying from their disease [30].
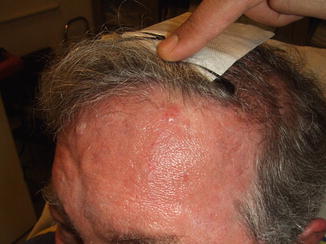

Fig. 8.3
One week after initial diagnosis, a 1 cm biopsy of a left forehead nodule anterior to hairline showing Merkel cell carcinoma
Additionally improved survival in patients with localized disease can be predicted by size of the tumor, with tumors less than 2 cm showing improved survival [44] (Fig. 8.4). Stage III patients who present with an unknown primary tumor have also been found to have a statistically significant better prognosis compared to Stage III patients with a known primary tumor [46, 47]. It is hypothesized that patients with an unknown primary tumor have most likely undergone spontaneous regression of their primary tumor. This regression may be attributable to a robust immune response and may partially explain their improved survival rates. A robust immune response has been shown in many different ways to improve survival. Detection of high antibody titers of MCPyV was a significant predictor for progression-free survival [48]. Additionally, tumors with a robust CD8+ and CD3+ intratumoral lymphocyte response show greatly better than expected MCC-specific survival [49, 50]. A recent study showed that p63 expression represents a strong risk factor for shortened survival, but this finding has yet to be confirmed by studies on larger cohorts [51].
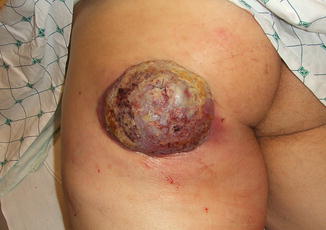

Fig. 8.4
A 59-year-old liver transplant recipient with rapidly enlarging 10 × 13 cm lesion on the left buttock
MCPyV-positive MCCs are less likely to metastasize and have a better prognosis than MCPyV-negative MCCs [52–55]. Additionally, MCPyV-positive tumors have fewer regional nodal metastases at time of diagnosis and are more likely to be located on the extremities than MCPyV DNA–negative MCCs [53]. Differences in tumor biology have been demonstrated and likely account for the differences in outcomes. For example, MCPyV-positive tumors cause carcinogenesis via pRB-mediated oncogenic pathways, while in contrast MCCs with few or no detectable copies of MCPyV depend on alternate oncogenic pathways, such as p53 [52].
Metastases most commonly occur through the lymphatic system, and nodal status is the best predictor of metastatic spread [56]. While distant metastasis is unusual at presentation, eventually as many as 35–50 % of patients will develop distant metastasis. Metastasis is most common to the lymph nodes, skin, lung, brain, bones, and liver [44, 57].
There are currently no evidence-based guidelines recommending how to follow patients for the development of progressive disease. Imaging modalities that have been utilized include (F-18-FDG)-PET scan, CT scan, and radiolabeled octreotide scintigraphy (OctreoScan), though the last is not considered standard of care [58]. FDG-PET has been shown to be better at detecting lymph node [59] and bone metastasis [60] than CT scan; however, it may miss liver metastasis, likely secondary to increased glucose metabolism in the liver, which can be detected with contrast-enhanced diagnostic CT [61]. Paulson et al. demonstrated that serology may be used to detect IgG antibodies to the MCPyV T antigen. These antibodies can be found in 40.5 % of MCC patients and tend to fall rapidly and stay low in patients who do not recur, and increase rapidly prior to detection of disease spread in patients with progression [62]. Additional studies are needed to further evaluate the role of MCPyV T antigen antibodies in monitoring for early signs of recurrent or progressive disease, as a target for future therapeutics.
8.8 Treatment
There remains a significant deficit of randomized control trials to evaluate therapeutic options for MCC. The annually published NCCN guidelines based on best available evidence and consensus recommendations from leading cancer centers can be utilized to guide management. Given the likelihood for an increased incidence and a more aggressive disease course, physicians need to take extra vigilance when caring for OTRs. Patients should have regular full skin and lymph node exams. These exams should be every 3–6 months for the first 2 years after diagnosis and every 6–12 months thereafter. Imaging studies are only needed as clinically indicated. Patients should be educated on performing self-skin examinations on a monthly basis. OTRs have an increased risk of all types of skin cancers, and daily sun protection should be emphasized. A multidisciplinary approach is needed to care for OTRs diagnosed with MCC, as they are likely to have an aggressive disease course. Close follow-up and co-management with the transplant team with possible reduction or revision of immunosuppression may lead to improved outcomes, though no specific data exists.
Currently, treatment is dictated by the clinical stage at presentation and parallels that used in the general population. Wide local excision with 2 cm margins is recommended, followed by histologic examination to confirm clearance of all margins. Margins of at least 2 cm should be obtained whenever possible, as this will decrease the risk for recurrence although it may not improve survival [63–65]. In patients who are not candidates for surgery, radiation can be used to gain local control, and in one report 4 patients who received radiation monotherapy were disease free at 5 years [66].
MCC is a highly radiosensitive tumor. Most studies have demonstrated a positive association between the addition of adjuvant radiation and improved locoregional control [67–72]. While adjuvant radiation may reduce the probability of regional recurrence, it has not been shown to improve overall survival [72].
Lymph node status should be addressed at the time of wide local excision with a sentinel lymph node biopsy (SLNBx). SLNBx has been shown to be a less morbid procedure than full lymphadenectomy and provides significant prognostic information [73, 74]. The histologic status of the SLN can predict the status of the entire lymph node basin that is at risk for metastases [75]. Patients with clinically localized disease and pathologically proven negative nodes have improved survival when compared to those who only undergo clinical nodal evaluation [44]. Patients with a positive SLNBx have been shown to have a three times higher risk of developing recurrent disease than patients with a negative SLNBx, and in one study SLNBx changed the stage of one-third of MCC patients by demonstrating clinically occult microscopic nodal disease, ultimately altering their treatment course [56]. In these patients with clinically node-negative disease, microscopic metastatic disease can take up to 8 months to become clinically apparent [76]. Proper identification and staging of these patients better directs algorithms for treatment of MCC.
Elective lymphadenectomy can be undertaken for treatment of SLN-positive patients and has been associated with improved disease-free survival, but not overall survival [42, 70]. It is important to remember that this procedure comes along with a small but real risk of morbidity including infection, pain, and lymphedema. One large study found that in lymph node–positive disease irradiation to the primary lymph node basin is comparable to surgical outcomes with no detectable difference in overall survival and can be considered as a treatment option in patients with positive lymph nodes [77].
While MCC is generally believed to be a chemotherapy-sensitive neoplasm, no consensus as to chemotherapy treatment regimen exists. Additionally, it is rarely used in the setting of immunosuppression due to increased morbidity/mortality from marrow suppression and sepsis. Agents used to treat MCC are similar to those used to treat small cell lung carcinoma and include platinum-based agents, cyclophosphamide, doxorubicin, vincristine, prednisone, bleomycin, 5-fluorouracil, and various combinations of the above. Most chemotherapeutic regimens provide good initial regression of the lesion; however, recurrences occur commonly within the first 4–15 months following treatment [78]. The benefits of chemotherapy should be weighed against the risks, especially in elderly patients with multiple comorbidities and in immunosuppressed patients. In particular, one retrospective analysis linked patients who received adjuvant chemotherapy to a worse overall survival compared to patients who did not receive chemotherapy [45]. Additionally, there are no established second-line treatments for patients who progress while on chemotherapy. There is a resounding need for novel, biology-driven therapies for this disease.
8.9 Targeted Molecular Therapies
Octreotide, a somatostatin analog, has been shown to have antiproliferative effects on neuroendocrine tumor cells, possibly through the inhibition of tumor angiogenesis (Table 8.2). Because as many as 90 % of MCCs demonstrate somatostatin-2 receptors, octreotide is a promising therapy [79]. Additionally, radiolabeled octreotide scintigraphy may be utilized to detect the presence of these receptors to determine if a patient is likely to have a clinical response. There are two favorable cases reported in the literature of complete remission after somatostatin analog therapy with sustained remissions demonstrated at 10 and 17 months, respectively [80, 81]. Notably, very little toxicity was noted with the use of these medications. Complications reported include carcinoid syndrome and abnormal urinary 5-hydroxy-indoloacetic acid secretion. Disease showed stabilization for at least 6 months (range 1–32 months) [82]. A phase I trial of the somatostatin analog, pasireotide, by the National Institute of Health is currently ongoing.
Table 8.2
Targeted molecular therapies
Treatment | Mechanism | Current research | Results |
|---|---|---|---|
Octreotide | Somatostatin analog | Phase I trial underway with pasireotide by NIH | Median survival time 22 months with disease stabilization of at least 6 months |
Pazopanib | Receptor kinase inhibitor – targets VEGF and PDGF receptors | One case report | Complete remission of primary lesion partial remission metastatic lesions; 4 months till progression |
YM-155 | Downregulates survivin | Mouse xenograft model study | Halted the growth of MCV-positive MCC xenograft tumors and was nontoxic in mice, cytostatic as tumors regrew with withdrawal |
PI3K/AKT Inhibitors | Inhibits PI3K and AKT pathways which are upregulated | In vitro studies | Presence of a PIK3CA-activating mutation was associated with sensitivity to treatment in MCC samples with a specific PI3K inhibitor and to a dual PI3K/mTOR inhibitor |
Lorvotuzumab Mertansine | Maytansinoid microtubule assembly inhibitor fixed to CD56 monoclonal antibody | Phase I trial complete | 2 out of 12 patients with durable complete responses after treatment |
Pazopanib, a receptor kinase inhibitor that targets both vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF) receptors, has been hypothesized to inhibit tumor growth and angiogenesis. It is currently being used for both renal cell carcinoma and soft tissue sarcoma and is generally well tolerated. There is currently one case report in which a patient with MCC was treated with pazopanib and had complete regression of a scalp tumor and partial regression of pulmonary metastasis. Median time until progression was 4 months. Minimal adverse effects were seen, but dose had to be reduced from 800 mg daily to 400 mg daily after patient developed gallstone pancreatitis [83]. Additionally, a germ line mutation in the PDGFR-α gene that may serve as a marker for potential treatment response has been found in three patients [61].
Survivin is a cellular protein, frequently increased in MCC that is antiapoptotic to tumor cells. YM-155, a small molecule that downregulates survivin, has been found to be effective against other types of cancers in phase I studies and is considered to be safe with a generally low side effect profile [84]. Current studies of YM-155 in a mouse xenograft model of MCC appear promising with halted growth of MCPyV-positive MCC and no toxicity in mice; however, tumor regrowth occurred with withdrawal of the medication indicating it is likely cytostatic and not cytotoxic [85].
MCC has also been shown to be sensitive to PI3K/AKT inhibitors currently in early trials. PI2K/AKT pathway is upregulated in approximately 10 % of MCC tumors and is independent of tumor viral status [86].
Lorvotuzumab mertansine is comprised of a maytansinoid microtubule assembly inhibitor fixed with a humanized monoclonal antibody to CD56. Since CD56 is expressed on almost all MCC, it is hypothesized to target MCC. The phase 1 trial which included 12 MCC patients demonstrated durable complete responses in two of the MCC patients.
8.10 Immunotherapy
The discovery of MCPyV in MCC tumor cells opens the door to new, targeted therapeutic strategies such as immunotherapies (Table 8.3). As viral oncoproteins play a critical role in tumor development of MCPyV-positive MCC, exploiting these oncoproteins and disrupting their function may provide therapeutic benefit. For example, the T antigen–specific N-terminus domain shared by the small and large MCC T antigens may provide a target for vaccine or adoptive T-cell therapies [62]. Furthermore, the presence of viral peptides may be employed to stimulate the immune response against virus-infected tumor cells.
Table 8.3
Immunotherapies
Treatment | Mechanism | Current research | Results |
|---|---|---|---|
PD-1/PD-1L inhibitors | Blocks TCR signaling on lymphocytes | Phase I clinical trial ongoing | Complete or partial response in non-small cell lung cancer, melanoma, and renal cell cancer; responses lasted 1 year or more in patients with 1 year or more of follow-up |
Ipilimumab | Monoclonal antibody blocks CTLA-4 on T cells, increasing T-cell activation | clinical trials underway in MCC | Shown to improve survival in metastatic melanoma, average improved survival 6 months |
4-1BB | Co-stimulatory receptor on activated T cells | Phase I trials underway in advanced/metastatic solid tumors | In preclinical trials agonist antibody-activated NF-κB and induce downstream cytokine production, promote leukocyte proliferation, and inhibit tumor growth in a human xenograft tumor model |
Intratumoral IL-12 + electroporation | Allows delivery to tumor of IL-12 while avoiding systemic effects; biases towards a TH1 cytokine profile, induces IFN- γ production | Phase I trial in melanoma completed; phase II trials underway in MCC | Complete resolution of distant, nonelectroporated lesions in 10 % of metastatic melanoma; partial or stable response in 42 % and minimal side effects |
Systemic interferon (α and β) | Upregulate MHC-1 immune response | Case report – 2 patients | No regression and severe depression in patient 1; progression of all metastasis in patient 2 |
Intralesional interferon-β | Upregulate MHC-1 immune response | One case report and small-scale trial show local tumor regression | Local tumor regression in injected lesions |
One treatment exploits the upregulation of programmed death-1 (PD-1), an inhibitory cell surface receptor that blocks T-cell receptor (TCR) signaling on lymphocytes. It is commonly upregulated in patients with chronic viral infections and leads to the development of T-cell exhaustion [87]. Additionally, the administration of antibodies to block the interaction of PD-1 with its ligand has been shown to enhance T-cell response. In patients with MCC, PD-1 is upregulated on MCPyV-specific CD8 T cells when compared to control T cells [88]. Clinical trials for efficacy in MCC are currently ongoing. Durable responses have been shown in other tumors and appear to work the best in tumors that express PD-L1 [89]. Because of the immunosuppression and viral nature of many MCC, this is an encouraging therapeutic option.
Ipilimumab is a monoclonal antibody that blocks CTLA-4, an inhibitory receptor found on T cells. By inhibiting the inhibitor, ipilimumab is able to increase T-cell activation. It has been shown to improve survival in metastatic melanoma patients [90, 91] and may have future benefits in the treatment of MCC.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree