Fig. 7.1
High-risk primary SCC in an organ transplant patient
Moreover, immunosuppression after solid-organ transplantation was originally recognized in the dermatology literature as a risk factor for more aggressive SCC by Rowe in 1992 [18]. Others have confirmed that SCC in OTRs has an increased rate of local recurrence, metastasis, and morbidity [2, 12, 13]. The rate of metastasis of SCC in this population is 7–8 % as compared to 0.5–5 % in the general population [10, 18, 19]. Furthermore, metastasis in the setting of immunosuppression has been associated with a poor prognosis [20]. In-transit metastasis with SCC has also been described more commonly in OTRs and represents a unique, more aggressive presentation of SCC associated with a poor prognosis [21].
7.3 Immunosuppressive Agents
Lifelong immunosuppression prevents graft rejection, and improved regimens have opened the door to increased survival times in the field of organ transplantation. Most immunosuppressive agents, however, have also been associated with an increased risk of malignancy, notably skin cancers. Controversy exists as to which particular agents contribute most to the development of cutaneous malignancy, and there has been some suggestion that older agents including antiproliferative agents such as azathioprine and calcineurin inhibitors (e.g., cyclosporine) may contribute to the development of skin cancers more so than newer agents such as the mTOR inhibitor sirolimus [14, 22]. Little controversy, however, exists that the degree and duration of immunosuppression correlate with the development of aggressive SCC [2, 14]. Induction therapy with a lymphocyte-depleting agent or an interluekin-2 receptor antagonist is given just before, at the time of, or just following organ transplantation to prevent acute rejection [10]. This course is followed by maintenance immunosuppression, which is given in higher doses for the first 3 months posttransplantation when the risk of rejection is highest.
The earliest antirejection protocols from the 1970s traditionally included corticosteroids, azathioprine, and cyclosporine [2]. Azathioprine is a purine analog which acts as a prodrug for mercaptopurine and inhibits the proliferation of T and B cells [23]. Treatment with azathioprine, a potent photosensitizer, increases the skin’s susceptibility to damage from UVA radiation [10, 24]. Cyclosporine binds cyclophilin and inhibits calcineurin, preventing activation of interleukin-2 (IL-2) and inhibiting T cell formation [25]. Corticosteroids predominantly inhibit T cell proliferation [26]. Azathioprine, cyclosporine, and prednisolone have all been associated with an increased risk of cutaneous carcinogenesis with the proposed mechanism of decreased immune surveillance as well as possible direct carcinogenesis [2, 27–30]. Induction chemotherapy with OKT3, antithymocyte globulin, or monoclonal anti-IL2 receptor antibodies has also been associated with an increased risk of nonmelanoma skin cancers (NMSCs) [10, 31, 32].
Jensen et al. showed that kidney transplant recipients had a 4.2-fold increased risk of developing SCC while on triple immunosuppression (cyclosporine, azathioprine, and prednisolone) in comparison to those receiving only azathioprine and prednisolone [16, 17]. While Dantal et al. showed that 26 as compared to 17 patients in normal- and low-dose cyclosporine groups, respectively, developed at least one skin cancer correlating the degree of immunosuppression with the risk of malignancy [33]. Another large study in the United Kingdom found increased rates of cutaneous malignancies in renal transplant patients with poor renal function, requiring increased immunosuppression as compared to those receiving organs from a living donor who usually require lower regimens [15]. However, literature has suggested that cyclosporine, rather than diminishing the immune system’s response to carcinogenic cells, may in fact lead to non-immune-mediated mechanism of oncogenic development [34]. Hojo et al. found that cyclosporine may promote cancer development and progression through the induction of transforming growth factor-β [28].
Newer immunosuppressive regimens including agents such as mycophenolate mofetil, tacrolimus, and sirolimus have aimed at reducing the morbidity associated with long-term immunosuppression. Mycophenolate mofetil (MMF) inhibits de novo purine synthesis hindering T and B cell proliferation [35], while tacrolimus, a calcineurin inhibitor, also binds the FK-binding protein preventing the activation of IL-2 and therefore the proliferation of B and T cells [3]. Studies have shown a lower incidence of skin cancers in OTRs treated with tacrolimus in comparison to those treated with cyclosporine [36]. In animal studies of UVB-exposed mice, those treated with cyclosporine or tacrolimus developed larger tumors than vehicle-treated mice, while the addition of MMF to cyclosporine (but not tacrolimus) significantly reduced tumor size [37]. Other studies in mice however have shown that tacrolimus may have antiproliferative effects [38]. Currently, maintenance therapy with the calcineurin inhibitor tacrolimus over cyclosporine in combination with mycophenolate mofetil instead of azathioprine is recommended [10].
Sirolimus and everolimus are mTOR inhibitors that also bind to the FK-binding protein and may prevent angiogenesis resulting in decreased tumor size and production [3, 10, 11]. They are non-nephrotoxic and therefore are utilized in patients with cyclosporine and tacrolimus induced nephrotoxicity [10]. Studies have demonstrated a decreased risk of de novo malignancies, especially skin cancers, in transplant patients treated with sirolimus. Kauffman et al. showed that the incidence of de novo posttransplant malignancy with sirolimus or everolimus (another mTOR inhibitor) alone or of these agents in addition to cyclosporine or tacrolimus was 0.6 % versus an incidence of 1.81 % in patients treated with cyclosporine or tacrolimus [39]. In another study, patients on cyclosporine, steroids, and sirolimus who had their cyclosporine withdrawn 3 months posttransplant and their sirolimus troughs doubled while continuing on dual therapy with steroids showed reduced rates of both SCCs and BCCs [40]. Mathew et al. reported on the results from five trials involving sirolimus in posttransplant patients and found that 2 years after transplant those receiving sirolimus with cyclosporine had a lower incidence of skin cancer, while those receiving sirolimus as base therapy had no malignancies compared to 5 % in those being treated with cyclosporine, and that those who were trough level controlled on sirolimus with cessation of cyclosporine had a lower incidence of malignancy than those who remained on both agents [41]. Current recommendation is that sirolimus and everolimus should not be initiated until after graft function has been established [10].
7.4 Management
7.4.1 Standard Modalities
The gold standard of treatment in OTRs with SCC is surgical excision. It is also important to remember that SCC in OTRs on immunosuppression is considered a high-risk lesion correlated with increased incidence of local recurrence and metastasis [18]. Therefore, establishing which lesions may be at highest risk for metastasis or recurrence and prioritizing treatment of these lesions are important. In general, high-risk SCCs are characterized by large lesion size, rapid growth, aggressive pattern on histology, recurrence (such as those occurring in a scar), and location on the scalp, lip, or ear [2, 14, 18].
Mohs migrographic surgery has been shown to be associated with a lower frequency of local recurrence in the treatment of SCCs in OTRs and is considered standard of care for all high-risk SCCs, while Mohs or surgical excision may be used for low-risk tumors [18, 42]. Subclinical extension of tumor infiltrates has been shown in SCCs in OTRs, further strengthening the utility of Mohs surgery in this patient population (Fig. 7.2a, b) [43]. In addition, a review of OTRs presenting to a single center for Mohs micrographic surgery showed that OTRs have significantly more cutaneous malignancies referred for Mohs surgery with a reversal of the ratio of BCC to SCC as seen in the general population [44]. This study also showed that OTRs generally have smaller tumors than the general population reflecting a likely lower threshold for screening and referring these patients [44]. Standard excision with postoperative margin assessment may also be used with 6–10 mm margins rather than standard 4 mm margin in high-risk lesions when Mohs surgery is not available, the lesion is larger than what is permissible for removal under local anesthesia, or tissue conservation is not a priority [45, 46].
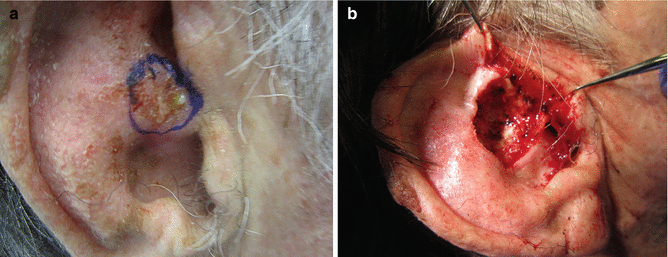

Fig. 7.2
(a) High-risk SCC lesion prior to surgical treatment. (b) High-risk SCC lesion with deep extension during Mohs excision
Additional modalities such as radiation therapy should only be considered in high-risk lesions if the tumor is inoperable the or patient is unable to undergo surgical excision [46]. However, radiation as an adjunctive treatment may be useful in cases involving perineural invasion [14, 46, 47].
Topical therapies with imiquimod or 5-fluorouracil may be used for in situ SCC lesions or malignant precursors such as actinic keratoses [14, 46]. Electrodessication and curettage may be used for SCC but should not be used for high-risk SCC as it may be associated with potentially higher rate of recurrence [18, 46, 48].
7.4.1.1 In-Transit Metastasis
In-transit metastasis is a unique presentation of SCC, most commonly seen in OTRs and associated with a poor prognosis in this population. Representing metastases to the surrounding skin via lymphatic spread, in-transit metastases are a therapeutic challenge in this patient population and must be considered carefully (Fig. 7.3). In one of the first reports on the characteristics of in-transit metastases in cutaneous SCC, a series of 21 patients with in-transit metastasis, (15 of which were OTRs) was reviewed finding that lesions were most often subcutaneous or dermal exophytic papules located in proximity (mean distance of 2.5 cm) to the primary lesion with multiple lesions also being common [21]. In-transit metastases in OTRs in this series was associated with a poor prognosis with only 33 % of patient achieving disease control at 24 months with traditional treatment modalities of Mohs surgical excision, standard excision, excision and radiation, radiation alone, or in a limited number of cases systemic or intralesional chemotherapy [21]. The mean time to development of in-transit metastasis in this review was 10 weeks, while the majority of regional metastasis occurs typically within one and a half years of the primary tumor being diagnosed [21, 49]. Additionally one-third of these patients were alive with either nodal disease or distant metastases at 24 months in comparison to 80 % of the non-transplant patients achieving control with 0 % disease-related mortality at 24 months [21]. As such, in-transit metastases should be handled with a multidisciplinary approach, with surgical excision in addition to consideration for adjuvant radiation therapy or sentinel node biopsy being made on a case-by-case basis [2, 14, 21]. In the experience of these authors, in-transit metastases are routinely removed with surgical excision of the new lesion and adjacent scar from the primary excision, and patients should subsequently be treated with radiation therapy (Table 7.1).
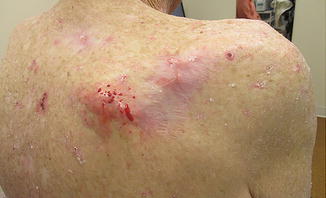

Fig. 7.3
In-transit metastatic SCC to the surrounding skin in a patient with a high-risk primary SCC
Table 7.1
Management of high-risk SCC in OTRs

7.4.1.2 “Field Cancerization”
The idea of “field cancerization” in OTRs has been used to describe the extensive areas of actinic damage found in this patient population that are often filled with multiple primary lesions and premalignant precursors or subclinical lesions (Fig. 7.4) [11]. The International Transplant Skin Cancer Collaboration guidelines for the treatment of SCC in OTRs recommend tangential excision followed by cautery for the treatment of in situ SCC for patients with multiple primaries and extensive field disease [46].
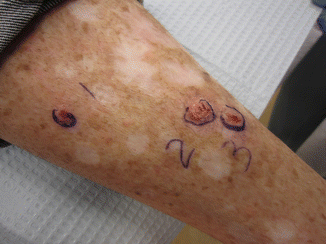

Fig. 7.4
Extensive field cancerization with background actinic damage and multiple primary lesions
Topical therapies and other destructive modalities, such as imiquimod, 5 % fluorouracil (5-FU), and photodynamic therapy, may also be used in concert with excision for patients with extensive areas of malignant and premalignant damage [14, 22]. Imiquimod, approved by the Food and Drug Administration for the treatment of genital warts, actinic keratoses, and superficial basal cell carcinomas, is a nonspecific immunomodulator that has been shown to be safe for use in OTRs on systemic immunosuppression [42, 50]. Topical 5-FU works via inhibition of thymidylate synthase which inhibits DNA synthesis and is the most widely used topical agent for extensive epidermal dysplasia though few studies have evaluated its efficacy in the OTR population [51]. In one study that examined the combination of imiquimod with 5-FU for biopsy-proven in situ SCCs in OTRs, clearing was reported and it was suggested that imiquimod may improve the efficacy of 5-FU [52]. Interestingly, in a small trial comparing 5-FU to photodynamic therapy—which involves the application of photosensitizer such as methylaminolaevulinate or aminolevulinic acid followed by phototherapy—photodynamic therapy was more efficacious and preferred by OTRs for the treatment of epidermal dysplasia extensive field damage [24].
7.4.2 Retinoid Therapy
Retinoids have also been reported as effective chemoprophylaxis for the prevention of skin cancers in OTRs. In this regard, acitretin is preferred over isotretinoin due to a more favorable side effect profile [14]. In a series of five heart transplant recipients with multiple NMSCs, the addition of low-dose acitretin (10 or 25 mg/day) in all five patients resulted in a reduction in the development of premalignant actinic keratoses [53]. In another study of low-dose acitretin (0.3 mg/kg/daily), 12 of 16 patients showed significant reduction in new carcinomas for up to 4 years of treatment of patients with five or more SCCs prior to acitretin therapy benefiting most [54]. In a larger series of 44 renal transplant patients, the addition of 30 mg/day of acitretin decreased the incidence of SCC as well as reducing the occurrence of actinic keratoses, with the effect being most pronounced in those patients with a history of NMSCs [55]. Systemic retinoid therapy, therefore, should be considered in any patient who develops 5–10 SCCs per year, with a lower threshold considered in OTRs with extensive field damage or a history of more aggressive SCCs [42].
7.4.2.1 Sirolimus
Recent investigations into the use of the mTOR inhibitor sirolimus have suggested it may reduce cutaneous SCCs in OTRs. Hoogendijk-van den Akker et al. examined conversion to sirolimus in renal transplant patients with a history of at least one biopsy-proven SCC and found a nonsignificant 24 % reduction in risk of SCCS, with a significant 50 % reduction in the first year [56]. Similar studies by Campbell et al. and Euvrard et al. showed that conversion from calcineurin inhibitors to sirolimus decreased the incidence of new SCCs [57, 58]. Importantly, the studies by Hoogendijk-van den Akker et al. and Euvrard et al. found that the greatest reduction in the development of SCCs occurred early on in the first 1–2 years following conversion. Additionally they found a nonsignificant reduction in the number of subsequent SCCs in patients who had numerous SCCs prior to conversion suggesting that conversion to sirolimus should potentially be considered earlier than the current International Transplant Skin Cancer Collaborative guidelines which recommend conversion only following the development of numerous SCCs, a high-risk SCC, or an SCC with in-transit or metastatic disease [56, 58, 59].
7.4.2.2 Capecitabine
Capecitabine is a precursor of 5-fluoruracil and is approved for the treatment of breast cancer and colorectal cancer [42]. A prospective study of oral capecitabine plus subcutaneous interferon-alfa in four patients with advanced cutaneous SCC showed complete remission in two patients with partial remission in a third patient and the fourth patient dying from unknown causes [60]. A subsequent study with lower-dose capecitabine that is used in colorectal carcinoma in three OTR patients who developed more than one SCC per month showed significant improvement in the development of SCCs, as well as inflammation and regression of clinically apparent premalignant actinic keratoses [61]. Additional studies are needed to measure the utility of this agent in OTRs for the prevention of SCCs and premalignant lesions.
7.4.2.3 Cetuximab
Limited data has also shown that high-risk cutaneous SCC with more than three high-risk features or very high-risk cutaneous SCC displaying lymphovascular, perineural, parotid, periorbital, cartilaginous, or bony invasion; in-transit metastasis; or metastasis that are treated with adjuvant cetuximab in combination with surgery may have improved outcomes [62]. Diffuse alveolar damage that proved fatal, however, has been reported in two lung transplant patients, and cetuximab should therefore not be used in this patient population [63].
7.4.2.4 Sentinel Lymph Node Biopsy
Sentinel lymph node biopsy (SLNB) of truly high-risk tumor cases while not standard of care has also been reported in the literature for surgical staging and may confer further prognostic value [2]. It has been suggested that SLNB may allow for the detection of subclinical nodal involvement with a recent study of SNLB in 24 patients with NMSCs (17 of which were SCC) showing a sensitivity of 88 % and a specificity of 100 % [64]. In this study, Wagner et al. found that seven patients had a tumor-positive node that was not clinically detectible [64]. In a series examining SLNB in SCCs on the trunk and extremities, Reschly et al. found that four of nine patients had positive nodal involvement, with two of these patients dying from metastatic disease within 2 years in comparison to all five of the node-negative patients being alive and well at a mean follow-up of 8 months [65




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






