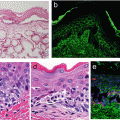
Fig. 15.1
Role of the immune system in obese versus lean adipose tissue. In obese adipose tissue, Th1-type cytokines such as IFN-γ stimulate M1 macrophage polarization. Mast cells are also increased in obese adipose tissues, contributing to insulin resistance. M1 macrophages produce pro-inflammatory cytokines such as TNF-α and IL-1β. In contrast, in lean adipose tissue, Th2 cells produce anti-inflammatory cytokines such as IL-4 and 13, which promote alternatively activated M2 macrophage polarization. M2 polarization is also induced by eosinophils via IL-4. M2 macrophages secrete other anti-inflammatory signals such as IL-10, which maintains insulin sensitivity in lean adipose tissue. Macrophages are bone-marrow–derived myeloid cells. Hence, both M1 and M2 macrophages express the myeloid cell surface markers F4/80 and CD11b. However, only the M1 population expresses the marker CD11c
The local environment of adipose tissues may influence the ratio of M1/M2 macrophages. M2-predominant ATMs are typically seen in normal lean individuals. On the other hand, obesity promotes infiltration of macrophages and induces a shift in the ATM balance towards the M1 phenotype. In fact, obesity shifts the ATM M1/M2 ratio from 1:4 in normal mice to 1:1.2 in obese mice [23]. This shift is evident in the expression level of CD11c on ATMs, a marker for M1 macrophages [24]. Flow cytometric analysis revealed that >90 % of recruited monocytes become CD11c+ M1 macrophages in obese adipose tissues [24].
15.2.4 Adipocyte-Resident Tregs
As discussed above, obesity is a kind of chronic, low-grade inflammation of adipose tissue that promotes insulin resistance and type-2 diabetes. These findings raise the question of how adipocyte inflammation can escape the powerful mechanisms of cells and molecules normally responsible for guarding against a runaway immune response. Feuerer et al. answered this question in a recent report in which they observed that CD4+ Foxp3+ regulatory T cells (Tregs) with a unique phenotype were highly enriched in the abdominal fat of normal mice, but their numbers were strikingly and specifically reduced in the abdominal fat of insulin-resistant obese mice [12]. Loss-of-function and gain-of-function experiments revealed that these Tregs influenced the inflammatory state of adipose tissues and, thus, insulin resistance. Cytokines differentially synthesized by fat-resident regulatory and conventional T cells directly affected the synthesis of inflammatory mediators and glucose uptake by cultured adipocytes [12]. These observations suggest that harnessing the anti-inflammatory properties of Tregs to inhibit the mechanisms seen in metabolic syndrome may have therapeutic potential.
15.3 Inflammatory Mediators from Adipose Tissues
In 1964, D. Ogston and G. M. McAndrew reported increased fibrinogen levels in plasma in obese subjects [31]. This was the first report to describe a correlation between inflammation and obesity. Nowadays, the hypothesis that adipose tissues have an endocrine function [1] is strongly supported by the findings that adipocytes secrete a variety of mediators, termed adipokines, which are involved in the inflammatory network (Fig. 15.2). These mediators include adiponectin, leptin, PAI-1, IL-6, and TNF-α [54]. Interestingly, although a higher expression level of IL-6 was found in visceral fat as compared with subcutaneous fat, no significant regional variation has been reported in TNF-α production. Notably, not only increased cytokine production but also impaired cytokine catabolism seem to be determinants of obesity-related inflammatory status [28].
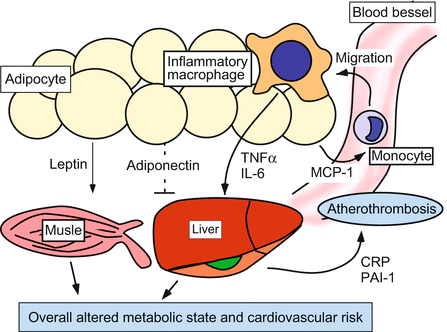

Fig. 15.2
Adipose tissue as an endocrine and paracrine organ. Obesity is associated with increased serum adipokine levels. TNF-α and IL-6, as well as leptin, induce insulin resistance in skeletal muscles and liver synthesis of pro-clotting factor that result in increasing risk of cardiovascular failure. In contrast, adiponectin mediates protective effects in obesity-related metabolic and vascular diseases. PAI-1 impairs fibrinolysis and enhances the risk of atherothrombosis
15.3.1 Adiponectin
Adiponectin is an anti-inflammatory cytokine produced mainly by adipocytes. Low serum levels of adiponectin have been reported in several chronic diseases such as obesity and psoriasis [3]. In patients affected by inflammatory diseases, high levels of cytokines such as TNF-α and IL-6 may reduce adiponectin production [3]. Adiponectin levels are inversely correlated with serum CRP levels in obese individuals. Moreover, reduced levels of adiponectin are associated with insulin resistance, impaired endothelium-dependent vasodilatation, impaired ischemia-induced neovascularization, and diastolic heart failure. Thus, adiponectin mediates protective effects in obesity-related metabolic and vascular diseases [3].
15.3.2 Leptin
Leptin is one of the main adipose-derived cytokines. Leptin has been investigated primarily for its role in controlling energy homeostasis by regulating appetite [33]. Leptin is also important for cell-mediated immunity. Previous studies have shown that CD4+ T cells are hyporeactive in leptin-deficient mice [22]. Congenital leptin deficiency in humans results in lower frequency of blood CD4+ T cells as well as impaired T-cell proliferation and production of cytokines such as IFN-γ, and this hyporesponsiveness can be restored by exogenous leptin [11]. Leptin appears to enhance Th1 and suppresses Th2 immune responses. In vitro, leptin acts on naive T cells, increasing their IL-2 secretion and proliferation, as well as increasing IFN-γ production by memory T cells [22]. Leptin is required for the induction and progression of autoimmune encephalomyelitis in mice [25], and it was recently shown that leptin inhibits the proliferation of regulatory T cells [10]. Leptin also activates macrophages by promoting IL-1β, IL-6, TNF-α, and IL-12 production [26]. Thus, elevated leptin levels may lead to enhanced Th1 type immune responses due to diminished regulatory T cell activity.
15.3.3 Resistin
Resistin was originally discovered in mouse adipocytes and assigned a key role in the induction of murine insulin resistance [45]. Human adipocytes, however, do not produce resistin [30], but resistin is produced by cells in the SVF, particularly by macrophages [9]. Resistin production is increased in the subcutaneous adipose tissues of obese individuals compared with lean individuals [38], but there is only a weak correlation between body mass index (BMI) and serum resistin levels [46]. Resistin is also produced by peripheral blood monocytes, and is particularly enhanced during their differentiation into macrophages [21, 32]. Resistin stimulates macrophages to produce inflammatory cytokines such as TNF-α, IL-1β, IL-6, CXCL8, and IL-12 [40], and conversely, TNF-α, IL-1β, IL-6, and lipopolysaccharides stimulate resistin production [21], suggesting a vicious cycle-type role of resistin in pathogenesis.
15.3.4 PAI-1
Another remarkable obesity-related abnormality is the increased circulating level of PAI-1 [2], the most important inhibitor of plasminogen activation. PAI-1 is correlated with all components of the insulin-resistance syndrome, and its level significantly decreases with weight loss [27]. Thus, the increased PAI-1 levels observed in obesity, by impairing fibrinolysis, enhance the risk of atherothrombosis. In addition, obesity and metabolic syndrome appear to be associated with other hemostasis alterations, such as enhanced platelet activity [2].
15.3.5 IL-6
Adipose tissue also produces a large amount of IL-6 [29]. The metabolic role of IL-6 is not fully understood; mice deficient in IL-6 develop mature-onset obesity and carbohydrate and lipid dysmetabolism [48]. On the other hand, mice chronically exposed to IL-6 develop insulin resistance [20]. Thus, this cytokine is likely a critical metabolic modulator. Obesity is associated with increased secretion of IL-6, and therefore higher hepatic release of acute-phase reactants, especially CRP [54]. CRP levels are higher in obese than in nonobese individuals, directly correlating with the waist-to-hip ratio even after adjustment for BMI [47]. CRP also directly correlates with other cardiovascular risk factors and increased cardiovascular risk in the absence of acute inflammation.
15.3.6 TNF-α
Not only ATMs but also adipocytes produce TNF-α. In obese subjects, a 7.5-fold increase in TNF-α secretion from adipose tissues was observed compared to lean subjects [19]. TNF-α has manifold effects on inflammation and metabolism. In psoriasis patients, TNF-α exerts pro-inflammatory activity on the synovial tissue and endothelium [35], contributing to oxidative stress and leucocyte recruitment into atherosclerotic plaques. Moreover, it induces insulin resistance in skeletal muscles, liver synthesis of pro-clotting factor and lipoprotein lipase inhibition, and increases serum levels of atherogenic lipoproteins [37]. Thus, TNF-α contributes significantly to atherosclerotic lesion development, especially by promoting the expression of adhesion molecules on endothelial cells, the recruitment and activation of inflammatory cells, and the initiation of the inflammatory cascade inside the arterial wall [36, 41].
15.4 Obesity and Psoriasis
15.4.1 Role of Obesity in Psoriasis Patients
The association between psoriasis and obesity was the subject of a recent evidence-based review [6]. A key question is whether obesity causes or is a consequence of psoriasis. Childhood-onset obesity may particularly predispose an individual to both psoriasis [5] and psoriatic arthropathy [42], which suggests a genetic link. Perhaps the best evidence that obesity can cause psoriasis is the fact that bariatric surgery can produce a rapid remission of psoriasis. In addition, there is increasing evidence that progressive weight loss can induce significant improvements in the severity of psoriasis [16]. However, it could be argued that a lack of physical activity, due either to the cosmetic impact of psoriasis or to the locomotor effect of psoriatic arthropathy, might predispose an individual to obesity [39].
15.4.2 Adipokines in Psoriasis Patients
As discussed above, ATMs produce TNF-α, as well as other cytokines involved in the pathogenesis of psoriasis such as IL-1, IL-6, IL-17, and IFN-γ [6, 43]. These adipokines, as well as leptin, are recruited and stimulated in obesity and may have an autocrine and paracrine effect on nearby skin [6] (Fig. 15.3).
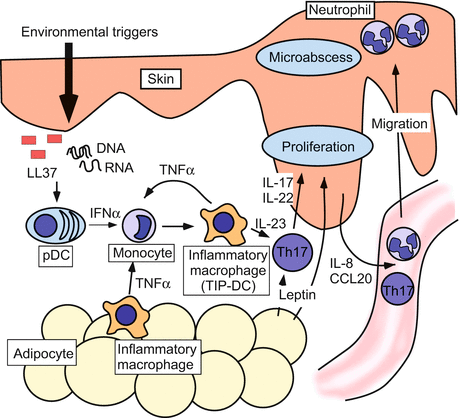

Fig. 15.3
Obesity and psoriasis. This schema depicts the association between psoriatic skin lesion and adipokines. Environmental stimuli trigger keratinocytes to produce self-DNA/RNA and LL37 complexes, which stimulate the synthesis of IFN-α by plasmacytoid DCs (pDC). IFN-α, in association with TNF-α, activates inflammatory macrophages. In obese individuals, TNF-α production is likely to be enhanced by ATMs and adipose tissues. Activated inflammatory macrophages produce multiple cytokines that promote differentiation and expansion of Th17 cells. Th17 cytokines induce keratinocytes to produce CCL20 and IL-8, chemoattractants for CCR6-expressing T cells and neutrophils, thus promoting the accumulation of these cells in the psoriatic skin. IL-22 and leptin enhance keratinocyte proliferation
Leptin, produced by adipocytes, decreases regulatory T cells and is involved in inflammatory processes stimulating cytokine release [22], as well as its more established role in appetite suppression and metabolic control [33]. In fact, leptin levels have been shown to correlate with the severity of psoriasis [7]. On the other hand, elevated levels of resistin from ATMs lead to insulin resistance and upregulate the production of inflammatory cytokines including TNF-α[17]. Resistin levels are also increased in patients with psoriasis, correlating with obesity and increased severity of psoriasis [17]. In contrast, low serum levels of adiponectin have been observed in obese psoriatic patients as compared with the nonobese psoriatic patients [8, 34].
Thus, there is some indirect evidence that the immunological and metabolic alterations associated with obesity may be linked with the pathophysiology of psoriasis.
15.5 Conclusions
On a histological level, adipose tissue inflammation in obesity is associated with macrophage accumulation. It is interesting to note that many cellular and biochemical components of the immune system that normally protect the host from foreign pathogens, such as macrophages and Toll-like receptors, also play a pathologic role in obesity-related inflammation. Overall, adipose tissue inflammation in obesity demonstrates the fact that the immune system and the metabolism are highly integrated.
References
1.
2.
Alessi MC, Juhan-Vague I (2008) Metabolic syndrome, haemostasis and thrombosis. Thromb Haemost 99:995–1000PubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree