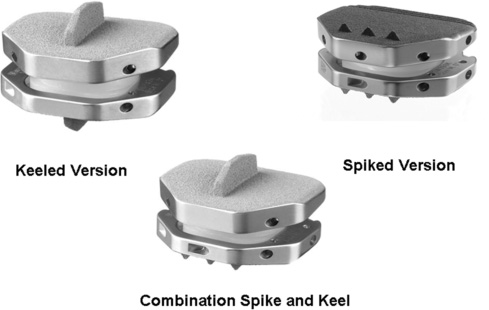
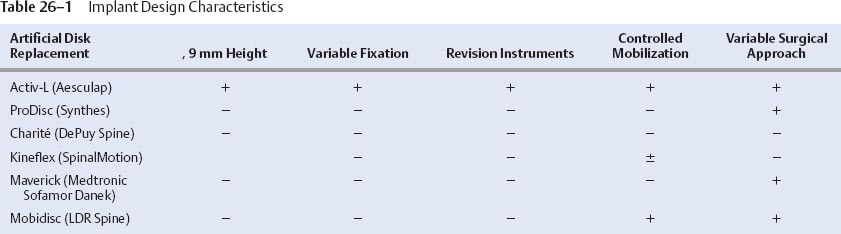
26 Anterior-Posterior Implant Insertion Several innovative and promising nonfusion spinal implant devices and accompanying techniques have recently been proposed for the treatment of debilitating axial diskogenic low back pain.1–14 Over the past Design improvements for the next generation of prostheses include those as outlined in Table 26–1. Material and biomechanical properties should be optimized to minimize wear debris, prosthetic designs should allow for ease of insertion via multiple angles, and surgical revision instrumentation should be available. In addition, the implant should provide maximal end plate coverage with minimal or no end plate recontouring, and the implant height should be minimized to protect neurological structures while concurrently allowing ease of implant delivery at single or multiple levels. The Activ-L prosthesis was designed to satisfy all of the foregoing next-generation requirements for lumbar ADR. We emphasize the concept that both implant design and insertional technique should be harmonious and considered as equally vital and important when comparing one implant with another. The indications for Activ-L intervertebral lumbar disk arthroplasty include severe lumbar diskogenic back pain as a result of lumbar spondylosis with or without radiculopathy. Clinical findings should be closely correlated with radiological imaging findings, including magnetic resonance imaging, standing plane x-rays, diskography, and computed tomography (CT) to assess the integrity of facet joints and the pars interarticularis. Other sources of back pain such as facet disease, myofascial syndromes, inflammatory arthritic conditions, and others should be excluded. The patient should have had at least 6 months of conservative measures prior to pursuing lumbar intervertebral total disk arthroplasty. Contraindications to this procedure include chronic infections such as periodontal sources of infection, urinary sources of infection, and chronic skin ulcerations. Patients with a body mass index greater than ~34 should be counseled extensively in an attempt to achieve a body mass index of less than or equal to 30 before proceeding with any intervertebral disk arthroplasty procedure. Patients should be appropriately screened and tested for osteoporosis prior to ADR surgery. Figure 26–1 Activ-L lumbar disk replacement. The Activ-L implant consists of three primary components (Fig. 26–2A,B). The superior and inferior components are composed of a three-metal alloy (cobalt, chromium, and molybdenum). The superior component is composed of a solid one-piece design with a central keel or three anterior spikes and is available in two lordotic angles—6 and 11 degrees. The superior component has a concave polished surface that articulates with the second component, an ultra high molecular weight polyethylene inlay (available in 8.5,10,12, and 14 mm). This inlay rests in the third (i.e., inferior) component, which is composed of a solid one-piece design with a central keel or three anterior spikes. The inferior component also has a highly polished surface that permits translation in the anteroposterior (AP) direction only during flexion and extension (Fig. 26–3). The S size permits for 1.5 mm of polyethylene inlay translation in the AP direction. The M, L, and XL sizes permit 2 mm of AP translation. None of the implants permit medial-lateral translation of the inlay. Four implant sizes are available: S, M, L, and XL (Fig. 26–4). The anteroposterior dimension of the S size is 26 mm. The medial-lateral dimension is 31 mm. The XL size is 33 mm in the AP dimension and 40 mm in the medial-lateral dimension. In terms of anatomical sizing, the AP dimension of each vertebral end plate must be at least 26 mm to permit the use of the Activ-L implant. The medial-lateral dimensions of the end plate must be at least 31 mm also, to allow for successful implantation. These measurements can be best evaluated on axial CT scan images, which are parallel to the intervertebral disk space in question. Both the superior and inferior components are plasma sprayed with a pure titanium coating as well as a thin layer (20 m m) of dicalcium phosphate dehydrate (m -CaP) to enhance bony ingrowth. Instrumentation for the Activ-L surgical procedure includes a set of disk space mobilization/distraction instruments (curettes, distractors, and disk space bullet distractors). Trial implant end plates for end plate sizing are available and can be placed directly on a mechanical distractor and intervertebral space sizer (Fig. 26–5). A midline pin marker can be placed to assist in midline accuracy. When an oblique insertion is desired, a radiolucent marker with radiopaque midpoint alignment markers is available to assist in midline marking in the AP and lateral projections (Fig. 26–6). Figure 26–2A,B Components of Activ-L disk replacement. Figure 26–3 Polyethylene (PE) inlay translates 2 mm in anteroposterior direction producing more physiological motion of vertebral segment. The end plates of the Activ-L implant are available in either keeled or spiked versions. These end plates can be mixed (superior keeled with inferior spiked or vice versa). When a keeled implant technique is desired, a protected chisel is available to cut the keel groove. The chisel is available in a double- or single-chisel design.
Activ-L Lumbar (Aesculap) Total
Disk Arthroplasty
 Description of System Components
Description of System Components
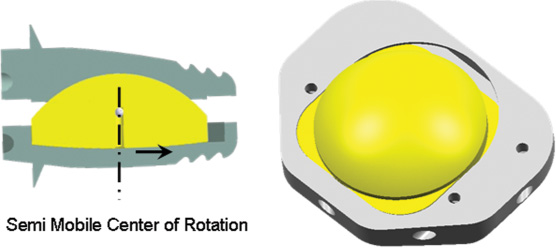
 decades, the early focus has been in the utilization of total disk arthroplasty. Biomechanically, there are presently three main types of artificial disk replacements (ADR): unconstrained, semiconstrained without translation, and, the next generation, semiconstrained with translation. This last type of motion we term mobilization. A more recent design, the Activ-L (Aesculap, Inc., Tuttlingen, Germany) (Fig. 26–1), allows for mobilization as well as implant stability and variability, uncomplicated prosthetic implantation, and revision instrumentation.
decades, the early focus has been in the utilization of total disk arthroplasty. Biomechanically, there are presently three main types of artificial disk replacements (ADR): unconstrained, semiconstrained without translation, and, the next generation, semiconstrained with translation. This last type of motion we term mobilization. A more recent design, the Activ-L (Aesculap, Inc., Tuttlingen, Germany) (Fig. 26–1), allows for mobilization as well as implant stability and variability, uncomplicated prosthetic implantation, and revision instrumentation.
Indications
Description of System Components
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


 Indications
Indications Operative Technique
Operative Technique Tips and Pearls
Tips and Pearls Complications
Complications Conclusion
Conclusion