Chapter 7 Tissue Assessment, Decision Processes, and Operative Planning
Background and Importance of Tissue Based, Quantitative Systems and Decision Processes
Preoperative Parameters and the Scientific Validity of Breast Augmentation Studies
Scientific validity of any prospective or retrospective study in breast augmentation requires that a study address variables that affect outcomes, defining those variables preoperatively, collecting objective, quantified data, and analyzing that data to test a hypothesis. A consistent weakness of most published studies in breast augmentation is a failure to define critical variables preoperatively and collect objective data pre- and postoperatively that relate to those variables. More than 53 variables affect the outcome of every breast augmentation. Table 7-1 lists surgeon and implant variables that exist in every breast augmentation.1
Table 7-1 Variables that impact outcomes in breast augmentation
| Clinical and tissue variables | Surgeon variables |
|---|---|
| Genetic factors | Incision approach |
| Hormonal factors | |
| Pregnancy history | |
| Nursing history | Pocket dissection method |
| Age | Pocket dissection technique |
| Medications (especially birth control pills and hormones) | Degree of tissue trauma |
| Skin and subcutaneous tissue thickness | |
| Skin compliance | |
| Postoperative drug regimen | |
| Size of periprosthetic pocket | |
| Shape of periprosthetic pocket | |
| Patient arm position | |
| Implant fill volume | |
| Air in implant | |
| TOTAL TISSUE VARIABLES: 31 | TOTAL SURGICAL VARIABLES: 22 |
| TOTAL VARIABLES, TISSUES AND SURGICAL: 53 | |
To be scientifically valid, any study that compares methodologies, surgical techniques, or implants must prospectively create comparative cohorts of patients that acknowledge and control for the 53 variables listed in Table 7-1
To be scientifically valid, any study that compares methodologies, surgical techniques, or implants must prospectively create comparative cohorts of patients that acknowledge and control for the 53 variables listed in Table 7-1. Regardless of the methods, techniques or implants the study attempts to compare, conclusions are scientifically invalid if the investigator does not preoperatively define two groups of patients, with each of the two groups having similar variables to those listed in Table 7-1. Addressing a small percentage of these variables is not adequate to validate conclusions—the two groups must have similar age, pregnancy, and tissue characteristics, quantified objectively, to achieve scientific validity.
Impact of Surgeon Education on Patients’ Experience and Outcomes
Surgeon education in breast augmentation and many other surgical disciplines is based on a preceptor model. Resident plastic surgeons learn breast augmentation principles and techniques from their professors and attending physicians. In many academic plastic surgery programs, resident experience in breast augmentation is limited, and attending academic surgeons may perform very few breast augmentations compared to plastic surgeons in private practice. When academic and private practice surgeons who teach residents refer to breast augmentation as a “simple” operation, it is not surprising that for more than three decades, reoperation rates of 15–20% in just 3 years2–4 following breast augmentation have changed minimally, and for many patients, the patient experience (recovery and outcomes) remains largely unchanged.
Redefining the Patient Experience in Breast Augmentation
The current decade has produced quantum improvements in surgeons’ approach to breast augmentation and has redefined the potential patient experience, outcome, and reoperation rates. Advances in tissue assessment, preoperative decision processes, surgical techniques, implant devices, and postoperative management now allow surgeons to routinely enable patients to return to full normal activities within 24 hours following breast augmentation with an overall reoperation rate of 3% over 7 years postoperatively.5–7 For the first time in the history of Food and Drug Administration (FDA) premarket approval (PMA) studies of breast implants, refined decision processes, methodologies, and techniques produced a zero percent reoperation rate in 50 consecutive patients in an FDA study of Inamed/Allergan Style 410 form stable, cohesive gel anatomic breast implants.8 These methods, which have redefined the surgeon and patient experience in breast augmentation, are peer reviewed and published in the journal Plastic and Reconstructive Surgery, and are available to surgeons worldwide. The specific tissue assessment, preoperative decision processes, and operative planning methodologies are the subject of this chapter.
Evolution of Preoperative Planning and Implant Selection Methods
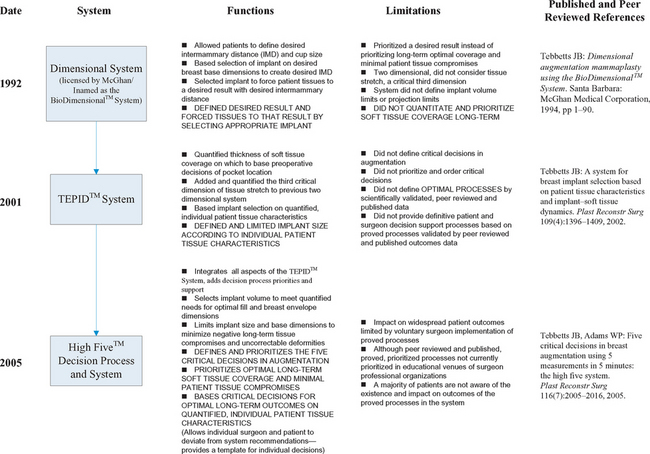
Table 7-2 summarizes the evolution of quantitative, tissue based systems and decision process support for patient assessment and critical decisions in breast augmentation since their inception in 1992.
Table 7-2 Evolution of quantitative, tissue based systems and decision process support for patient assessment and critical decisions in breast augmentation.
Objectives and Limitations of the First Generation Dimensional System
To attempt to increase the reliability and predictability of results to patients, in 1990 the author developed a dimensional system that enabled a more quantitative method of assessing a patient’s breasts and selecting appropriate implants to deliver the patient’s desired result. This first dimensional system was published in a monograph in 1994,9 and was licensed by McGhan Medical Corporation as the BioDimensional™ System and subsequently distributed worldwide. During the past decade, the BioDimensional™ System has become the most widely accepted and used dimensional system worldwide by providing surgeons with a more objective, quantitative, and scientific approach to breast evaluation and decision making in breast augmentation.
When first introduced in 1994, the BioDimensional™ System9 allowed patients to define their desired intermammary distance by displacing the breasts medially, then using measurements of the existing breast width and the desired breast width to determine the base width of the implant required to deliver the patient’s desired result. The original dimensional system functioned in two dimensions (base width and height), and focused on forcing tissues to a desired result (delivering the desired base width to achieve the patient’s goals for desired intermammary distance). This system is still in use as the Allergan BioDimensional™ System. This first generation dimensional system produced a paradigm shift in surgeons’ approach to patient assessment and decision making in breast augmentation. For the first time, surgeons could design a desired result using a quantitative assessment and decision making methodology instead of relying on bra stuffing, photographs, or subjective cup size methods to define objectives.
An additional decade of clinical experience and longer-term followup of larger numbers of patients have pointed out limitations of this first generation system: (1) the system prioritized achieving a specific result instead of prioritizing optimal soft tissue coverage long-term (did not include specific, quantifiable parameters that mandate optimal tissue coverage, (2) the system and current revisions by other surgeons have no guidelines or restrictions on volume limits, (3) the system is two dimensional, omitting a critical third dimension—tissue stretch—that affects decisions about optimal volume for a specific patient’s envelope and provides information about risks of excessive stretch with bottoming or traction rippling, and (4) the system was originally designed to be specific to one manufacturer’s family of anatomic implant products.
Priorities for the Next Generation Measurement and Decision Process System
To avoid implant palpability, visibility, visible traction rippling, and extrusion risks long-term, the surgeon must prioritize optimal, long-term soft tissue coverage of the implant and preservation of the patient’s existing breast parenchyma long-term, above all other considerations. Some of the most preventable complications of breast augmentation occur when surgeons and patients fail to prioritize optimal soft tissue coverage of the implant or decide to select implants with excessive size or projection that stretch, thin, and compromise tissues, and increase risks of parenchymal atrophy long-term.
Principles of Breast Aesthetics and Implant–Soft Tissue Relationships
Specific characteristics and relationships define an aesthetic breast. Every breast has a specific amount of skin that defines the dimensions—base width, stretch, and height—of the skin envelope. The skin envelope has specific tissue characteristics—base width, thickness, and stretch—that determine how much the envelope will stretch in response to adding fill (an implant). A funnel analogy is helpful when discussing these issues with patients. If the surgeon could pour filler into a funnel in the top of the breast, the lower breast would fill first until it reached its limit of anterior stretch, then the middle portion of the breast would fill, and finally the upper pole of the breast would fill to create an optimal breast contour and optimal transition of the breast from the chest. If the surgeon adds inadequate fill to any breast, the lower breast will be fuller compared to the middle or upper breast. When the breast is full with an optimal contour, adding additional volume creates excessive fullness in the upper breast, desired by some patients and surgeons, but also adding weight that ultimately causes more stretching of the lower envelope and loss of upper fill.
The wider the base width of a patient’s parenchyma, the longer the nipple-to-inframammary fold measurement for optimal aesthetics. Stated another way, the optimal position of the inframammary fold depends on the width of the breast. If the breast is excessively wide compared to the nipple-to-fold measurement, the breast appears boxy. If nipple-to-fold distance is too great compared to the width of the breast, the breast appears narrow, tubular, or ptotic. When increasing the width of the breast during augmentation, the optimal level to set the inframammary fold intraoperatively depends on the planned base width of the postoperative breast.
Two Basic Approaches to Breast Augmentation
No system is without tradeoffs. Even with a system that allows tissue dimensions and stretch to define volume requirements, some wide, parous breasts with minimal to moderate parenchyma require very large implants in order to adequately fill the envelope for an optimal aesthetic result. In these cases, patients must be aware that the amount of volume required for an optimal result may add enough weight to cause negative consequences to their tissues over time such as tissue stretch and thinning, parenchymal atrophy, visible or palpable implant edges, visible traction rippling, and ptosis. Some of those tissue consequences may be irreversible.
Tissue Coverage Priorities and Measurements
The system should set a quantified criterion to suggest additional muscle coverage (e.g. STPTUP < 2 cm) by placing the implant in either a traditional partial retropectoral or a dual plane (dividing origins of pectoralis along the inframammary fold) position. The system should also set a quantified criterion that suggests preserving muscle origins of the pectoralis intact along the inframammary fold when patient tissues along the fold are exceedingly thin (e.g. STPTIMF < 0.5 cm), choosing a traditional subpectoral pocket instead of a dual plane pocket that divides pectoralis origins along the inframammary fold.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree