Cutaneous Follicle Center Lymphoma
Cutaneous follicle center lymphoma is defined as the neoplastic proliferation of germinal center cells confined to the skin. The pattern of growth can be purely follicular, purely diffuse, or mixed. This lymphoma is listed as a specific entity in the World Health Organization (WHO) Classification of Tumours of Haematopoietic and Lymphoid Tissues [1].
The concept of primary cutaneous follicle center lymphoma as a morphologic spectrum including also cases with diffuse patterns of growth has been acknowledged only recently in general lymphoma classifications. In the lymph nodes, the large cell variant is classified as diffuse large B-cell lymphoma, and in the past the cutaneous diffuse variant was classified similarly. It may be that other extranodal follicle center lymphomas besides the cutaneous ones have analogous biological and prognostic features, different from those observed in nodal cases. In fact, it has been suggested that the model of a follicle center lymphoma that includes the diffuse variant may be useful to describe some extranodal diffuse large B-cell lymphomas characterized by an indolent course and by phenotypic features similar to those of cutaneous follicle center lymphoma, and that the concept of cutaneous follicle center lymphoma should be expanded to encompass some large cell lymphomas at extranodal sites [2].
Complete staging investigations must be performed in all patients, as secondary cutaneous manifestations of nodal follicular lymphoma may present with exactly the same clinicopathologic features as primary cutaneous cases [3]. In this context, it should also be underlined that positivity or negativity for Bcl-2 cannot be considered as a surrogate of complete staging investigations.
In the lymph nodes, rare cases of so-called “lineage plasticity” of follicle center lymphoma with transdifferentiation into Langerhans cell tumor have been observed (see also Chapter 1) [4]. Similar cases have never been reported in the skin.
Clinical Features
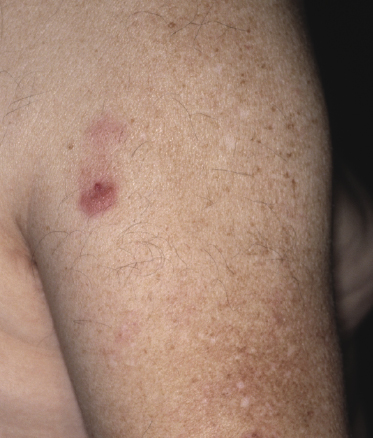
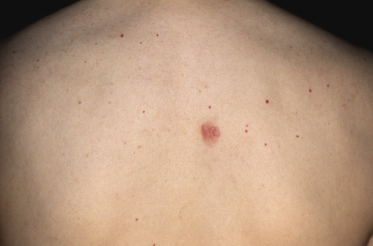
Patients are adults of both genders. Onset in children has been reported, but is exceptional [5, 6]. Cutaneous follicle center lymphoma presents with erythematous papules, plaques, and tumors, usually nonulcerated (Figs 11.1 and 11.2). Lesions are frequently clustered at a single site but may be multiple at different sites, mostly on the head and neck and the trunk. Small solitary plaques, especially those located on the back and upper extremities, may show a hint of the perilesional erythema that is characteristic of larger lesions located on the back (“Crosti’s lymphoma,” see below) (Fig. 11.3).
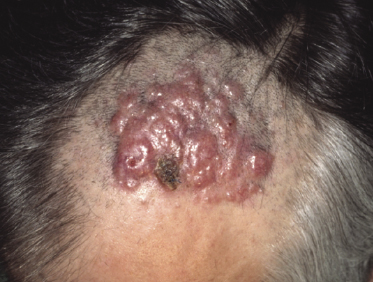
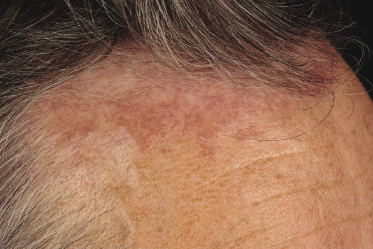
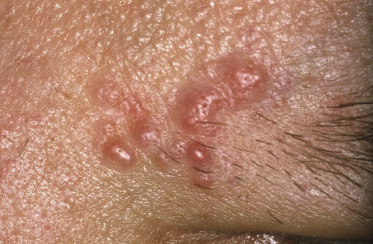
Follicle center lymphoma on the head may be clinically deceptive and present only with small papules, area of diffuse, slightly indurated erythema, or alopecia (Figs 11.2 and 11.4) [7]. Alopecia may also be observed as a specific manifestation of extracutaneous lymphoma (one such case was included as a teaching case in the 3rd edition of this book [8]). Other cases show miliary and/or agminated papules, resembling rosacea or arthropod bite reactions (Fig. 11.5) [9], or rarely rhinophyma [10]. Miliary and agminated lesions have been observed also in secondary cutaneous cases [11], again underlining the fact that primary and secondary cutaneous follicle center lymphoma may show identical clinical features.
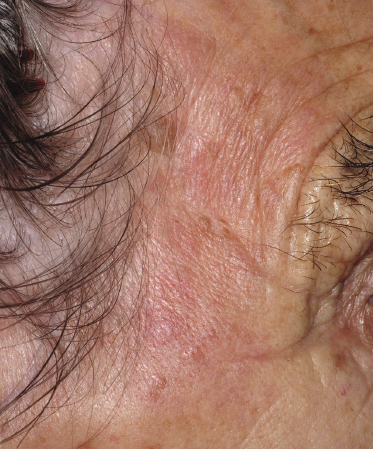
A distinct clinical presentation with plaques and tumors on the back surrounded by erythematous macules and papules expanding centrifugally around the central tumors has been described in the past as “reticulohistiocytoma of the dorsum” or “Crosti’s lymphoma” (Fig. 11.6) [12]. Early lesions of Crosti’s lymphoma show only small, circumscribed areas of erythema and/or small clustered papules and plaques, and may be very difficult to diagnose clinically (Fig. 11.7) [13].
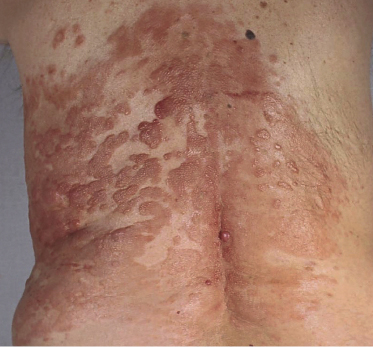
Although there are no clear-cut differences in clinical presentation between the diffuse and follicular variants of cutaneous follicle center lymphoma, cases with a follicular pattern have a predilection for the head and neck region, whereas tumors of Crosti’s lymphoma correspond in the majority of cases to a follicle center lymphoma with a diffuse pattern of growth.
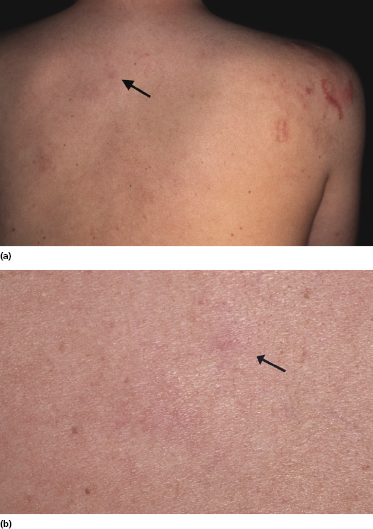
In several patients with the clinical presentation of Crosti’s lymphoma, small erythematous papules located far from the main lesions can be observed (Fig. 11.8) [13]. These papules represent early manifestations of the disease and reveal histopathologically specific features of follicle center lymphoma (Fig. 11.9). The question arises as to whether local radiotherapy is the more appropriate treatment modality for these patients and what the radiation field should be. The relatively high incidence of local recurrences observed in Crosti’s lymphoma treated by radiotherapy may be caused, at least in part, by the presence of early lesions far from the main tumor, which had not been identified clinically at the time of treatment planning and had not been included in the radiation field.
Some patients report that small papular lesions (particularly on the back) undergo spontaneous regression [13]. I have observed this phenomenon in some cases, and it may be more common than we currently realize.
Anetoderma has been observed rarely in cases of primary cutaneous follicle center lymphoma, and in some patients was associated with the presence of antiphospholipid antibodies [14]. Association with infections such as Borrelia burgdorferi, hepatitis C, or human herpesvirus 8 (HHV-8) has been described in sporadic patients, but does not seem to be an important etiologic factor for cutaneous follicle center lymphoma [15, 16]. In one patient the lesions arose at the site of previous radiotherapy for breast cancer [17].
Histopathology, Immunophenotype, and Molecular Genetics
Histopathology
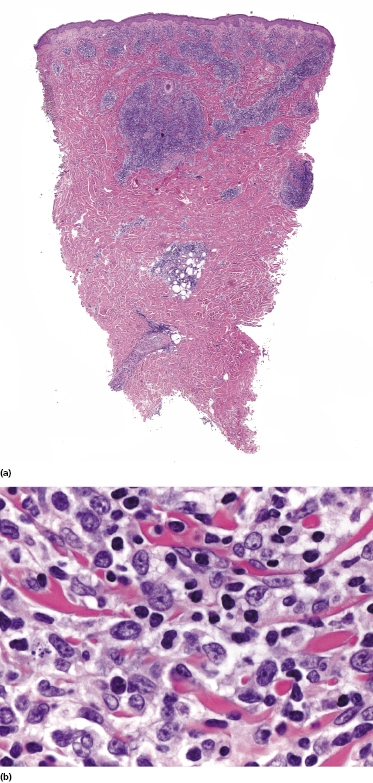
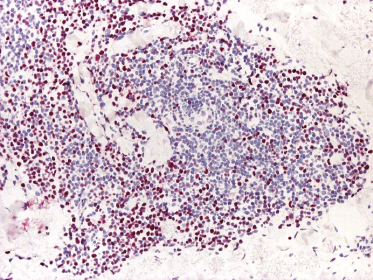
Well-developed lesions of cutaneous follicle center lymphoma with a diffuse pattern of growth involve the entire dermis, often extending into the subcutaneous fat (Fig. 11.10). They are characterized by a proliferation of medium and large cleaved cells (centrocytes) admixed with rounded cells with large, vesicular nuclei and nucleoli located close to the nuclear membrane (centroblasts) (Fig. 11.11). Small reactive lymphocytes are almost invariably admixed with the tumor cells, mostly dispersed through the infiltrate. When they are arranged in large aggregates, a biphasic pattern characterized by clusters of dark cells (corresponding to small reactive lymphocytes) and areas with clear cells (corresponding to the neoplastic cells) may be observed (Fig. 11.12). Small, isolated lymphoid follicles may be present focally in some cases with a predominantly diffuse pattern of growth (Fig. 11.12). These lymphoid follicles may be part of the neoplastic process or reactive.
Early lesions of follicle center lymphoma, diffuse type, are composed of relatively small, dermal lymphoid aggregates. There are variable numbers of medium and large cells, mostly showing morphologic features of centrocytes, admixed with many small reactive lymphocytes (Fig. 11.9) [13]. Clear-cut lymphoid follicles are not observed as a rule. This finding, together with the absence of follicular dendritic cells in about half of the specimens, suggests that many cases of early follicle center lymphoma, diffuse type, are characterized by a diffuse pattern of growth from the outset, never showing a clear-cut follicular arrangement [13].
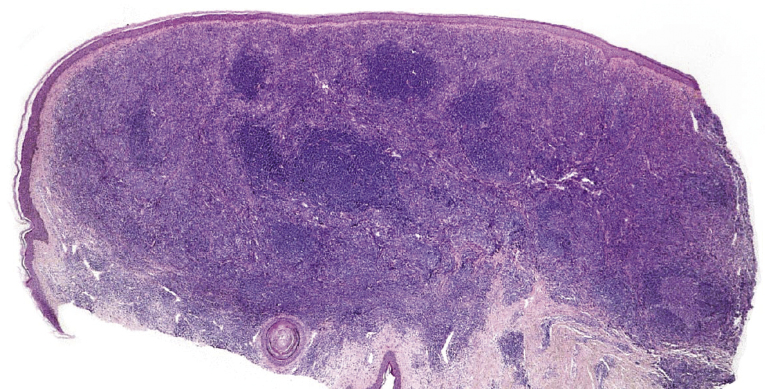
The histopathologic features of cases with a follicular pattern of growth consist of nodular infiltrates extending into the entire dermis, usually involving the subcutaneous tissues, characterized by a prominent follicular pattern (Fig. 11.13). The epidermis is spared as a rule. Neoplastic follicles in follicle center lymphoma show several morphologic abnormalities, such as a reduced or absent mantle zone, a reduced number or complete lack of tingible body macrophages, and a monomorphous appearance without a clear-cut distinction between dark and light areas (Fig. 11.14). These features are readily observed at low power and provide valuable clues for the diagnosis. Cytomorphologically, neoplastic follicles consist of small and large centrocytes admixed with centroblasts, often intermingled with reactive small lymphocytes. Small clusters of neoplastic cells can be found in the interfollicular areas as well.
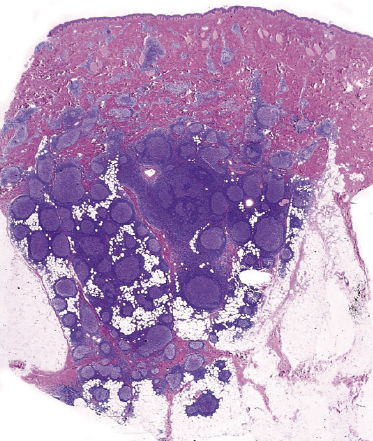
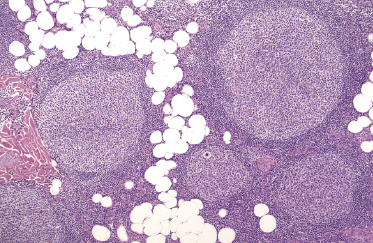
In some cases, cutaneous follicle center lymphoma with a follicular pattern of growth may show an “inversion” of the architecture of the lymphoid follicles, characterized by a pale area located at the periphery of the nodules (corresponding to neoplastic cells), surrounding a darker, central area (corresponding to reactive lymphocytes) (see Teaching case 11.1). These features are never encountered in reactive follicular infiltrates.
As already mentioned, follicular structures may be observed focally in some cases with a predominantly diffuse pattern of growth. In these cases, the follicles are usually visible at the periphery of the infiltrate with the diffuse pattern, which constitutes the majority of the lesion. Rarely, both patterns of growth (diffuse and follicular) represent approximately equal parts of one and the same tumor (Fig. 11.15).
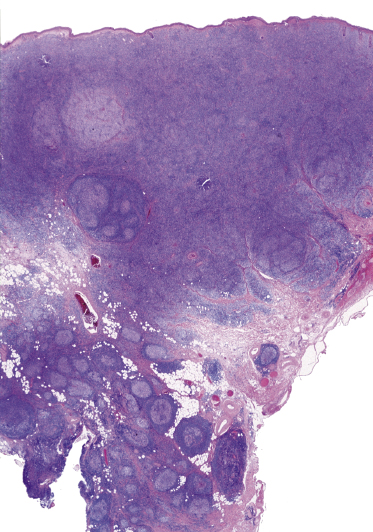
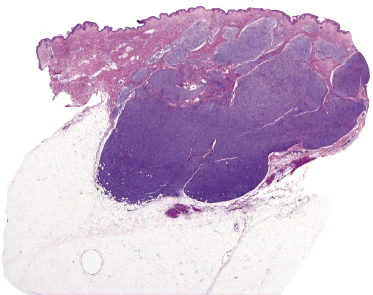
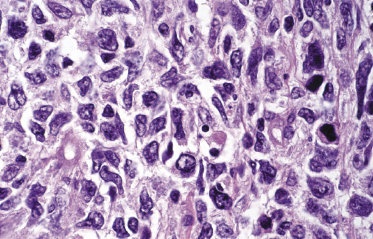
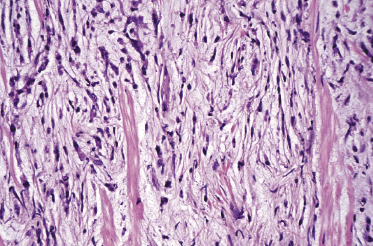
A morphologic variant of cutaneous follicle center lymphoma showing nodules of medium–large centrocytes admixed with some centroblasts without a prominent interfollicular infiltrate has been termed in the past “large cell lymphocytoma” (Fig. 11.16) [18, 19]. Another peculiar histopathologic variant is characterized by the predominance of spindle and bizarre cells, and has been termed “cutaneous spindle cell B-cell lymphoma” or “cutaneous sarcomatoid B-cell lymphoma” (Fig. 11.17) [20–25]. The bizarre cells are centrocytes that show different sizes and shapes. Although these cases were previously interpreted as a variant of cutaneous diffuse large B-cell lymphoma, most of them represent examples of cutaneous follicle center lymphoma, diffuse type [22]. At extracutaneous sites, too, the spindle cell variant is characterized by genotypic and phenotypic markers of germinal center cell origin [26]. Sclerosis and myxoid areas may also be observed in cases of cutaneous follicle center lymphoma, particularly in the spindle cell variant of the disease (Fig. 11.18).
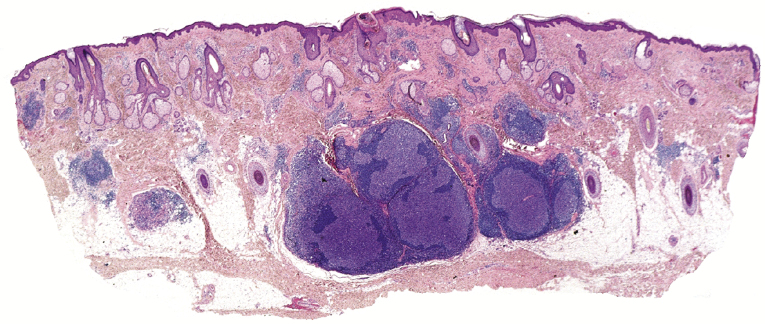
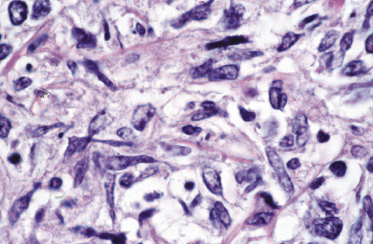
In the lymph nodes, cases of follicle center lymphoma may uncommonly show plasmacytic differentiation, posing differential diagnostic problems with marginal zone lymphoma (MALT-lymphomas). A clonal relationship between neoplastic lymphocytes and plasma cells has been demonstrated in some of these cases, confirming that they are morphologic variations of follicle center lymphoma [27]. I have observed rarely similar cases in the skin, with clonal neoplastic plasma cells within a lesion of otherwise typical follicle center lymphoma.
Immunophenotype
In both the diffuse and follicular variants of cutaneous follicle center lymphoma neoplastic cells are positive for B-cell markers, such as CD20 and CD79a, and for Bcl-6. Most cases showing a diffuse pattern of growth are negative for CD10, but positivity may be observed (Fig. 11.19). A network of CD21+ follicular dendritic cells is present in all cases with follicular pattern, but almost never in tumoral lesions of cutaneous follicle center lymphoma, diffuse type. In early lesions of the diffuse type clusters of CD21+ cells are found focally in about half of the cases. In lesions showing both patterns (follicular and diffuse), CD21+ follicular dendritic cells are usually located at the periphery of large areas with a diffuse pattern of growth (Fig. 11.20).
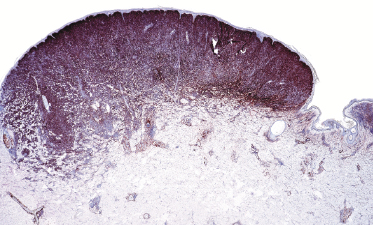
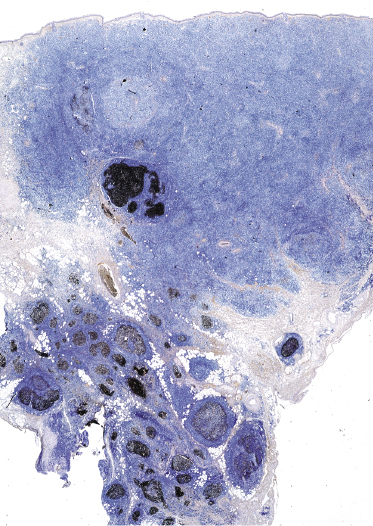
Early lesions of follicle center lymphoma, diffuse type, are characterized by small, irregular clusters of Bcl-6+ cells (usually negative for Bcl-2) (Fig. 11.21). There are no recognizable follicles in these cases and the Bcl-6+ cells are often arranged at the periphery of the lymphoid infiltrates. The presence of these clusters is strongly suggestive of cutaneous follicle center lymphoma, as they are never encountered in reactive infiltrates or in other types of low-grade cutaneous B-cell lymphoma.
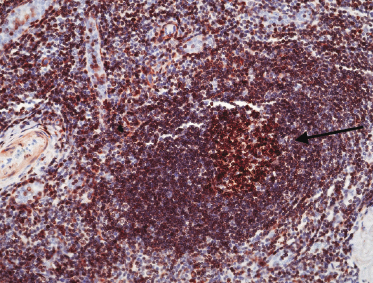
Cases of follicle center lymphoma with a follicular pattern of growth are positive for markers of germinal center cells such as CD10 and Bcl-6 (Figs 11.22a and b) [28]. Staining for CD10 may be negative in some cases. The presence of small clusters of CD10+ and/or Bcl-6+ cells outside neoplastic follicles can be observed in a proportion of these cases. This phenomenon, caused by the active “migration” of neoplastic follicular cells from the follicle to the interfollicular area and back, has been described in nodal follicular lymphomas as well, and is not observed as a rule in reactive lymphoid infiltrates, thus being virtually diagnostic of follicle center lymphoma. A good diagnostic clue for cutaneous follicle center lymphoma with a follicular pattern is provided also by the staining for proliferating cells (Ki67/MIB-1). Reactive germinal centers show a high degree of proliferation (more than 90% of cells), whereas neoplastic follicles often show a proliferative fraction of less than 50% of the cells (Fig. 11.22c) [28]. There is no aberrant expression of CD5 or CD43 by the neoplastic B lymphocytes.
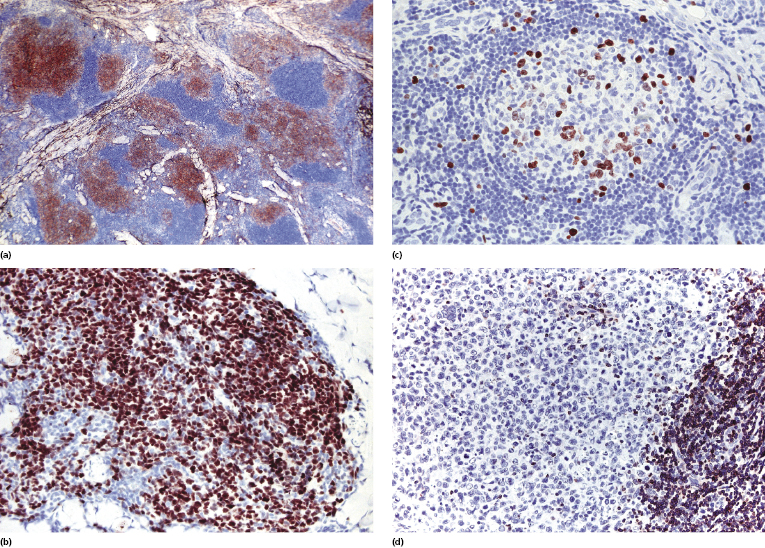
Besides Bcl-6 and CD10, other markers can be used in order to confirm germinal center differentiation of neoplastic cells. Paired box gene (PAX)-5 and interferon regulatory factor (IRF)8 are usually expressed, but other B cells are positive as well. HGAL and LMO2 have been reported as useful markers for detection of the interfollicular and diffuse components [29]. In my experience, these markers do not offer information beyond that provided by Bcl-6, but may be helpful in cases with equivocal Bcl-6 staining.
Although conflicting statements have been proposed, analysis of published reports shows that Bcl-2 expression can be found only in a minority of cases of primary cutaneous follicle center lymphoma [28, 30, 31]. Bcl-2 expression is present in 10–15% of cases within a small minority of the follicular cells, and only very rarely in the whole neoplastic population (Fig. 11.22d). On the other hand, when present Bcl-2 positivity in lymphoid follicles is virtually diagnostic of follicle center lymphoma and rules out a reactive process (Fig. 11.23). It should be underlined that the follicular mantle is always Bcl-2+ and that only the expression within germinal center cells should be considered evidence of malignancy. Positivity for Bcl-2 is not synonymous with cutaneous spread by nodal lymphoma, but positive cases should be staged very carefully.
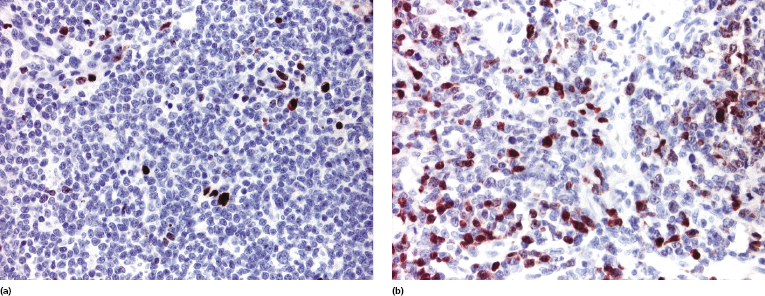
The multiple myeloma oncogene-1 (MUM-1) is positive only in a minority of cells (<30%) in cutaneous follicle center lymphoma (Fig. 11.24a) [32]. In rare cases the percentage of positive cells may be higher, but the homogeneous positivity observed in diffuse large B-cell lymphoma, leg type, is never found (Fig. 11.24b). Negativity for Bcl-2 and MUM-1 represents the most important criterion for differentiation of cutaneous follicle center lymphoma, diffuse type, from cutaneous diffuse large B-cell lymphoma, leg type (see also the section on differential diagnosis of cutaneous follicle center lymphoma, diffuse type, from cutaneous diffuse large B-cell lymphoma, leg type, in this chapter).
The detection of an immunoglobulin light chain restriction is difficult in paraffin sections, but can be observed on snap-frozen tissue sections. In situ hybridization is more reliable than conventional immunohistochemistry for detection of immunoglobulin light chains on paraffin sections, but negativity for both kappa and lambda is common in cutaneous cases. As already mentioned, rarely a small monoclonal population of plasma cells belonging to the same neoplastic clone as the follicular cells may be observed. Care should be taken to distinguish these cases from cutaneous marginal zone lymphoma, where monoclonal expression of immunoglobulin light chain kappa or lambda represents the most useful diagnostic criterion (see Chapter 12). Presence of other histopathologic and phenotypic features of follicle center lymphoma allows proper classification of these cases.
Molecular Genetics
Cutaneous follicle center lymphoma shows a monoclonal rearrangement of the immunoglobulin (Ig) genes in the majority of cases. Analysis of the molecular clones in different biopsies taken from various skin sites of involvement or at the same cutaneous site at different time points showed that the same clone is present in most cases. However, in a small percentage of patients different clones were detected in different specimens, suggesting the possibility that these cases represent multiple, unrelated primary cutaneous follicle center lymphomas [33]. Somatic hypermutations resulting in clonal evolution represents an alternative explanation of this finding [34].
Analysis of data from the literature clearly shows that the interchromosomal (14;18) translocation is extremely uncommon in primary cutaneous follicle center lymphoma, although the frequency varies in different studies (depending also on the method used for detection) (Fig. 11.25) [28, 30, 35]. Analysis of nodal t(14;18)-negative cases revealed rearrangement of BCL6 in about one-third of them [36], but data on cutaneous cases are lacking. Interestingly, genetic studies on nodal t(14;18)-positive and negative cases revealed that t(14;18)-positive cases show the signature of germinal center B cells, whereas activated B-cell-like, NFκB, proliferation, and bystander cell signatures were increased in t(14;18)-negative cases [37]. These signatures are different from the germinal center cell one observed in cutaneous cases of follicle center lymphoma, again pointing at different genetic backgrounds between cutaneous and nodal diseases.
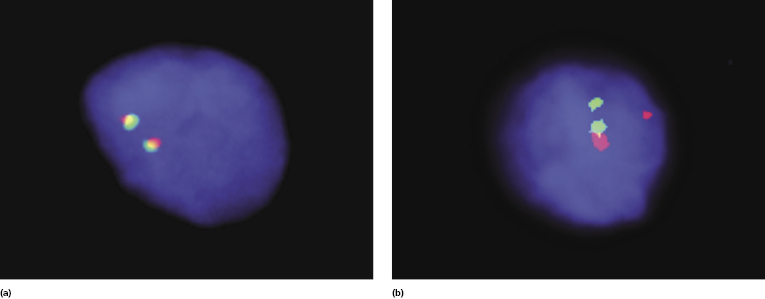
A t(12;21)(q13;q22) has been detected in one patient [38]. Gene expression studies using cDNA microarrays revealed that cases of cutaneous follicle center lymphoma have a germinal center cell signature [39, 40] and show hypermutated immunoglobulin variable genes with frequent use of the VH1-69 and VH4-59 segments [41].
Differential expression of a group of genes involved in regulation of lymphopoiesis and malignant transformation (polycomb group) was found in cutaneous B-cell lymphomas arising on the head and trunk as opposed to those located on the legs, confirming that these lymphomas represent distinct entities [42].
Differential Diagnosis of Cutaneous Follicle Center Lymphoma, Diffuse Type, from Cutaneous Diffuse Large B-Cell Lymphoma, Leg Type
Cutaneous follicle center lymphoma, diffuse type, and cutaneous diffuse large B-cell lymphoma, leg type, are both characterized by a predominant population of large B lymphocytes, thus creating problems in the differential diagnosis. Morphologically, the diffuse type of cutaneous follicle center lymphoma shows predominance of large cleaved lymphocytes, in contrast to cutaneous diffuse large B-cell lymphoma, leg type, where large round cells (particularly immunoblasts) are the majority [32]. Immunohistology reveals negativity for Bcl-2, MUM-1, IgM, and forkhead box protein 1 (FOX-P1) in cutaneous follicle center lymphoma, as opposed to positivity for these four markers in the vast majority of cases of cutaneous diffuse large B-cell lymphoma, leg type [32, 43]. Markers of germinal center cells such as Bcl-6 and less frequently CD10 are positive in cutaneous follicle center lymphoma, but may also be positive in diffuse large B-cell lymphoma, leg type, thus not providing helpful clues for the differential diagnosis. Molecular studies showed that neoplastic cells in the two subgroups have different gene signatures (cutaneous follicle center lymphoma: germinal center cell signature; and cutaneous diffuse large B-cell lymphoma, leg type: activated B-lymphocyte signature) [40].
It should be noted that cases of cutaneous follicle center lymphoma, diffuse type, arising on the legs have a bad prognosis, similar to that of cutaneous diffuse large B-cell lymphoma, leg type [32, 44]. The reason for this peculiar behavior is currently unclear. It may be that, in spite of predominant large cleaved morphology and/or negativity for Bcl-2, MUM-1, and FOX-P1, these rare cases represent in fact morphologic and/or phenotypic variations of diffuse large B-cell lymphoma, leg type. Be that as it may, patients with cutaneous follicle center lymphoma arising on the legs should be managed with the greatest caution.
Treatment
The best treatment for patients with cutaneous follicle center lymphoma is still a matter of debate. Most experts agree that aggressive strategies such as systemic chemotherapy should be reserved only for cases with extracutaneous involvement or with untreatable, generalized cutaneous disease (cases with generalized cutaneous disease, however, are very rare) [1]. A watchful waiting strategy may be considered, as the prognosis is excellent.
Local radiotherapy is still considered the first-line treatment [1, 45–48], but new data question the validity of this approach, particularly for lesions located on the back (see the section on clinical features in this chapter) [13]. Local radiotherapy is the best modality for solitary or clustered tumors. Surgical excision may also be considered for solitary lesions. The anti-CD20 monoclonal antibody (rituximab) has been used both systemically and intralesionally with good results [49–51], but in my experience recurrences are frequent when patients are treated with rituximab alone (recurrences in these cases may be CD20−). Rituximab can also be combined with systemic chemotherapy in patients with generalized skin or extracutaneous involvement and can be conjugated with radionuclides in order to provide selective irradiation of tumor cells.
Interferon-α (either systemically or intralesionally) has been used for treatment of patients with cutaneous follicle center lymphoma, sometimes associated with other treatment regimens and can be considered if alternatives are not feasible [48].
It has been suggested that disrupting the crosstalk between malignant B cells and accessory cells may be a novel strategy for treating low-grade B-cell malignancies (irrespective of the site of origin) [52], but at present there are no data on cutaneous follicle center lymphomas or other low-grade B-cell lymphomas of the skin.
Prognosis
The prognosis of patients with primary cutaneous follicle center lymphoma is very good, regardless of the pattern of growth [1, 28, 45, 53, 54]. In many cases lesions grow slowly over years or decades and only seldom spread to extracutaneous sites (Fig. 11.26). Although local recurrences can be frequently observed, extracutaneous involvement is uncommon. Involvement of the central nervous system has been observed rarely [55].
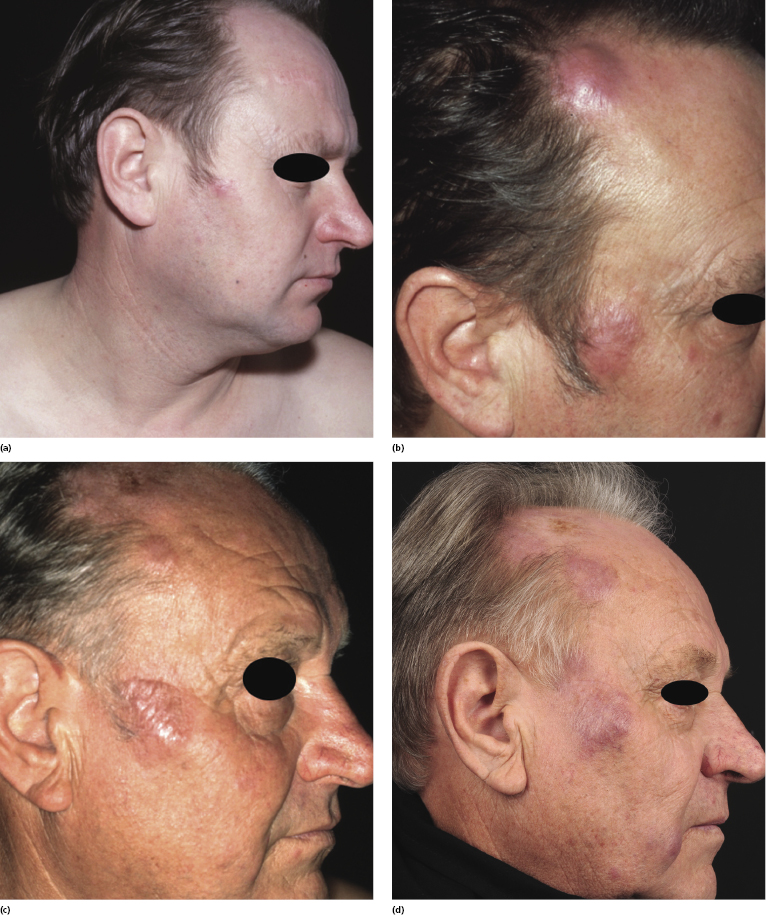
In rare cases, in spite of the absence of extracutaneous spread, local growth of tumors located on the scalp may cause infiltration of the underlying bone structures with osseous erosion and consequent meningeal exposure [56].
A prognostic index based on elevated LDH, number of lesions >2, and presence of nodular lesions has been suggested for indolent cutaneous B-cell lymphomas (cutaneous lymphomas prognostic index – CLIPi) [57]. However, in my experience the presence of more than two lesions and/or of nodular lesions does not have any impact on behavior; thus I do not think that this index adds any new information concerning prognosis of indolent cutaneous B-cell lymphomas, including cutaneous follicle center lymphoma.
As already mentioned, it is important to underline the fact that cases of follicle center lymphoma presenting clinically with lesions located on the legs and histopathologically with a diffuse pattern of growth may have a worse prognosis, and should probably be classified as diffuse large B-cell lymphoma, leg type [32, 44].

Full access? Get Clinical Tree


