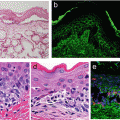
Fig. 6.1
Dendritic epidermal T cells (DETCs). Epidermal sheet from a normal adult mouse stained with an anti-γδ TCR monoclonal antibody (green, DETCs) and DAPI (blue, mainly keratinocytes)
Unlike conventional αβ T cells that express diverse TCRs and play a central role in adaptive immunity, tissue-resident γδ T cells express tissue-specific invariant or restricted TCRs and mount rapid, innate-like responses to tissue stress [10, 11]. Nevertheless, γδ T cells are not a functionally homogeneous population. Instead, there exist heterogeneous γδ T-cell subsets exerting a diverse array of functions in each tissue.
6.2 Murine γδ T Cells
6.2.1 Murine γδ T-Cell Subsets and Their Development
Several subsets of murine γδ T cells have been identified (Table 6.1). Murine γδ T-cell subsets have been classified based on the TCR Vγ (variable region of γ chain) and/or Vδ gene usage [11, 13], because TCR-defined γδ T-cell subsets differentially localize in specific tissues. These γδ T-cell subsets also exert distinct functions, although functional γδ T-cell subsets do not exactly overlap with the TCR usage [14, 15].
Table 6.1
γδ T-cell subsets
Mouse | TCR usage (Nomenclature According to Garman et al. [12]) | Characteristics |
|---|---|---|
DETCs | Vγ3Vδ1 | Development: early fetal thymus, Skint1-dependent, IL-15-dependent |
Localization: epidermis | ||
Phenotype: CD27− CD44+ CD62L− CD122+ SCART2− | ||
Functions: IFN-γ, IL-13, FGF7, FGF10, IGF1, cytotoxic, immunoregulatory | ||
IL-17–producing γδ T cells | Vγ4Vδ1 | Development: early fetal thymus (next to Vγ3Vδ1 T cells) |
Localization: vagina, uterus, tongue, peritoneal cavity | ||
Phenotype: CD27−CD44+ CD62L−CD122−CCR6+ SCART2+ RORγt+ | ||
Functions: IL-17A | ||
Vγ2-biased | Development: late fetal/perinatal thymus, TGF-β–dependent, IL-7–dependent | |
Localization: lymphoid tissues, dermis | ||
Phenotype: CD27−CD44+ CD62L−CD122−CCR6+ SCART2+ RORγt+ | ||
Functions: IL-17A | ||
NKT-like γδ T cells | Vγ1.1Vδ6.3/6.4 | Development: late fetal thymus |
Localization: liver, spleen | ||
Phenotype: CD27+ CD44+ CD62L−CD122+ PLZF+ | ||
Functions: both IFN-γ and IL-4 | ||
T10/T22-specific γδ T cells on the T10/T22-expressing background | Diverse (W…EGYEL motif in CDR3δ) | Development: mainly adult thymus |
Localization: spleen | ||
Phenotype: CD27+ CD44+ CD62L−CD122+ T-bet+ | ||
Functions: IFN-γ | ||
Naive γδ T cells | Mainly Vγ1.1 and Vγ2 | Development: mainly adult thymus |
Localization: lymphoid tissues | ||
Phenotype: CD27+ CD44−CD62L+ CD122− | ||
Functions: IFN-γ | ||
Intestinal γδ IELs | Mainly Vγ1.1 and Vγ5 | Development: neonatal thymus (first 3 weeks after birth), cryptopatches |
Localization: intestinal epithelium | ||
Phenotype: CD27+ CD122lo CCR9+ | ||
Functions: IFN-γ, FGF7, cytotoxic, immunoregulatory | ||
Human | Characteristics | |
Vδ1 T cells | Localization: skin (mainly dermis), intestinal epithelium, liver, lymphoid tissues | |
Antigen specificity: MICA/MICB, CD1c/CD1d-lipid | ||
Functions: IFN-γ, IGF1, cytotoxic | ||
Vγ9Vδ2 (Vγ2Vδ2) T cells | Localization: peripheral blood | |
Antigen specificity: phosphoantigens | ||
Functions: IFN-γ, IL-17A (CD161+ and CLA+ subsets), CTGF, FGF9, cytotoxic, APC function | ||
Murine γδ T-cell subsets expressing different TCRs develop in the thymus at different stages of ontogeny. T cells expressing an invariant (canonical) Vγ3Vδ1 TCR (nomenclature according to Garman et al. [12]; Table 6.2) are the first T cells to develop in the fetal thymus around day 14 of gestation [13]. Both Vγ3 and Vδ1 chains lack junctional diversity [13] due to the absence of terminal deoxynucleotidyl transferase (TdT) in fetal thymocytes. These Vγ3Vδ1 T cells migrate to the epidermis and become DETCs [17, 18]. Vγ3Vδ1 T cells are generated only in the early fetal thymus, and found only in the epidermis in adult mice [19, 20]. T cells expressing an invariant Vγ4Vδ1 TCR are the next to develop in the fetal thymus, and migrate to the vagina, uterus, tongue, and peritoneal cavity [21, 22]. Vγ2 T cells, which have diverse junctional sequences, develop in the thymus after these cells and reside mainly in the peripheral lymphoid tissues, but a subset also migrates to the dermis [3, 4, 23]. In the adult thymus, Vγ1.1 and Vγ2 T cells are predominantly generated, and emigrate mainly to the peripheral lymphoid tissues. This sequential development of murine γδ T-cell subsets is programmed at the level of Vγ gene rearrangement. Ordered Vγ gene rearrangement is determined by the location of Vγ genes in the TCRγ locus and by the altered accessibility of Vγ genes in the adult thymus [24].
Unlike conventional αβ T cells that differentiate into distinct effector/regulatory subsets during activation in the periphery, functions of murine tissue-resident γδ T cells are programmed during thymic development [11, 15, 25–27]. Maturation of Vγ3Vδ1 DETC precursors in the fetal thymus requires “positive selection,” which depends on TCR engagement with its ligand/agonist and interaction with the Skint1 molecule expressed on thymic epithelial cells [28–34]. Mature DETC precursors selected by the TCR ligand express skin-homing receptors and CD122 (IL-15 receptor β chain) [29, 34–38], which are crucial for their migration to and survival/expansion in the skin, respectively [34, 39–41]. Ligand-selected mature Vγ3Vδ1 thymocytes are CD27+ CD44+ CD62L−CD122+ and produce IFN-γ, although DETCs become CD27− [4, 42].
The CD27+ CD44+ CD62L− CD122+ phenotype is shared by natural killer T (NKT)-like γδ T cells that express restricted Vγ1Vδ6.3/6.4 TCRs and secrete both IFN-γ and IL-4 [43–45] and by T10/T22-specific γδ T cells that develop on the T10/T22-expressing background and produce IFN-γ [46]. Similar to DETCs, precursors of these γδ T-cell subsets receive strong TCR signals by recognizing the TCR ligands and are positively selected during thymic development [46–48].
IL-17-producing γδ T-cell subsets share a CD27−CD44+ CD62L−CD122−CCR6+ SCART2+ RORγt+ phenotype. IL-17-producing γδ T-cell subsets include Vγ4Vδ1 T cells residing in the vagina, uterus, tongue, and peritoneal cavity [22] and Vγ2-biased CD27−γδ T cells in the peripheral lymphoid tissues [49, 50] and in the dermis [3, 4, 23, 51]. Unlike ligand-selected IFN-γ-producing γδ T-cell subsets, precursors of IL-17–producing γδ T-cell subsets receive weak TCR signals by recognizing the TCR ligands or independently of the ligand recognition during thymic development [34, 46, 49, 52]. IL-17–producing γδ T cells expand in response to IL-7 [3, 53].
γδ T cells with a “naive” CD27+ CD44−CD62L+ CD122−phenotype residing in the peripheral lymphoid tissues can secrete IFN-γ upon activation [49]. They express diverse TCRs (mainly Vγ1.1 and Vγ2) [49]. Precursors of these γδ T cells may develop in the absence of TCR engagement and emigrate from the thymus as naive γδ T cells [49, 54, 55].
Intraepithelial lymphocytes (IELs) of the intestine contain γδ T cells expressing mainly Vγ1.1 and Vγ5 TCRs. The origin and development of intestinal γδ IELs are controversial [56]. Thymus exports γδ IEL precursors during the first 3 weeks after birth [57, 58], which may colonize cryptopatches in the intestinal lamina propria and develop into mature IELs extrathymically [59, 60]. Intestinal γδ IELs are CD27+ CD122lo CCR9+ [55], IFN-γ–producing, and cytotoxic T cells [56], but also have an immunoregulatory (suppressive) function and produce fibroblast growth factor 7 (FGF7 ), also called keratinocyte growth factor 1 (KGF1 ), to maintain tissue integrity [61].
6.2.2 DETCs
DETCs located in the basal epidermis have a dendritic morphology, and are in contact with neighboring keratinocytes and overlaying Langerhans cells through dendrites. DETCs uniformly express an invariant (canonical) Vγ3Vδ1 TCR [13] and recognize an as-yet-undetermined self ligand induced on stressed, damaged, or transformed keratinocytes through the TCR [62, 63]. In situ immunofluorescence staining with the soluble DETC TCR demonstrated that the DETC TCR ligand is undetectable in the normal epidermis, but upregulated on periwound keratinocytes rapidly and transiently after wounding [64, 65]. There is evidence suggesting that low levels of the DETC TCR ligand, which might not be detectable by immunofluorescence staining, are expressed in the normal epidermis [66–71]. A recent study showed that TCRs on DETCs are clustered and triggered at steady state in immunological synapse-like structures on the apical dendrites located near the keratinocyte tight junctions [72]. In response to tissue stress, these TCR-triggered proximal signals are relocated from the apical dendrites to the newly formed synapses [72].
DETCs also express several non-TCR stress receptors such as junctional adhesion molecule-like protein (JAML) [73], CD100 [74], and NKG2D [75, 76]. In addition to these coactivating/costimulatory receptors, DETCs constitutively express coinhibitory receptors including Ly49E, CD94-NKG2A [77], and E-cadherin [78]. Therefore, DETC activation is regulated by the balance between positive and negative signals provided through various coactivating, costimulatory, and coinhibitory receptors.
Upon activation, DETCs produce a variety of proinflammatory cytokines and chemokines, and may promote cutaneous inflammation [2, 79–81]. DETCs have been regarded as an IFN-γ-producing γδ T-cell subset, but can secrete IL-13 and trigger T helper 2 (Th2) -type responses [82]. DETCs also have an immunoregulatory function to downregulate cutaneous inflammation [83, 84].
Activated DETCs secrete FGF7/KGF1 , FGF10/KGF2 , and insulin-like growth factor 1 (IGF1) , and promote wound healing [85, 86]. DETCs recognize wounded keratinocytes through the TCR [64, 70], JAML [73], CD100 [74], and NKG2D [87, 88]. DETCs constitutively produce low levels of IGF1 in the normal, unperturbed epidermis, which may mediate maintenance of epidermal homeostasis by preventing keratinocyte apoptosis [86]. DETC-deficient mice have defects in the epidermal barrier function and epidermal structure [89, 90]. FGF7, FGF10, and IGF1, which are secreted by DETCs, are also known as the major hair growth regulators. DETC-deficient mice have defects in depilation-induced hair cycling (delay in anagen completion and acceleration of subsequent hair cycling) [91].
6.2.3 Murine Dermal γδ T Cells
Dermal γδ T cells are a newly discovered murine γδ T-cell subset [3, 4, 23, 51]. They are round or amoeboid in shape [3, 51], reside mainly in the superficial dermis, but are mobile [3, 51] and migrate to the lymph nodes at low rates [96]. Dermal γδ T cells express intermediate intensity of TCRs [3, 4, 51]. About 30–50% of dermal γδ T cells express Vγ2 TCRs [3, 4, 97]. Dermal γδ T cells express CCR6, SCART2, RORγt, and IL-23R [4, 23, 51] and secrete IL-17A upon IL-23 plus IL-1β stimulation or during cutaneous infection [3, 4, 51]. As do other IL-17–producing γδ T cells [14], dermal γδ T cells arise from the precursors that develop in the perinatal thymus [51]. Dermal γδ T cells express CD127 (IL-7 receptor α chain) [3, 51], and their development/maintenance depends on IL-7 but not IL-15 [3].
IL-17A secreted by dermal γδ T cells plays an important role in antipathogen responses during intradermal BCG infection [3]. IL-17–producing dermal γδ T cells may also mediate defense against cutaneous infection with Staphylococcus aureus [98], which has been attributed to DETCs [99, 100]. A recent study showed, however, that a subset of DETCs produces IL-17A upon TCR stimulation, which induces epidermal antimicrobial peptides and promotes wound healing [42].
Dermal γδ T cells can also produce IL-22 [4, 51] and are involved in the development of psoriasiform dermatitis in several different murine models [4, 96, 101–103].
Dermal γδ T cells induce hair follicle regeneration after wounding by producing FGF9, but the primary source of FGF9 was shown to be Vγ4Vδ1 T cells rather than Vγ2 T cells [97].
6.3 Human γδ T Cells
6.3.1 Human γδ T-Cell Subsets
Human γδ T cells are divided into two major subsets based on the TCR usage [26, 104] (Table 6.1). Vδ1 T cells are the predominant subset in the epithelial tissues. By contrast, most of the peripheral blood γδ T cells are Vγ9Vδ2 (also called Vγ2Vδ2) T cells. Although both subsets are implicated in anti-infection and antitumor immunity, they have distinct migratory capabilities and exert distinct functions according to the type of pathogens or tumors [105].
6.3.2 Human Vδ1 T Cells
Vδ1 T cells are the major human γδ T-cell subset preferentially residing in the epithelial tissues including the skin and intestine [8, 9, 106]. Human skin-resident Vδ1 T cells are present mainly in the dermis but also found in the epidermis [8]. They express skin-homing receptors CCR8 and cutaneous lymphocyte antigen (CLA) [8, 9].
The ligands for Vδ1 TCRs are largely unknown, but Vδ1 T cells were shown to recognize stress-induced self molecules such as MHC class I chain-related proteins MICA and MICB , which can also bind to NKG2D in addition to the TCR [107–109], and lipids presented by CD1c or CD1d [110–113].
Vδ1 T cells are IFN-γ-producing, cytotoxic T cells, and thought to mediate epithelial tumor surveillance [9, 114]. Indeed, Vδ1 T cells are frequent in the lymphocytes infiltrating solid tumors. Vδ1 T cells also accumulate in the skin lesions of leprosy patients [115] and expand in the peripheral blood during cytomegalovirus (CMV ) infection [116, 117].
6.3.3 Human Vγ9Vδ2 T Cells
The majority of γδ T cells in the adult human peripheral blood express Vγ9Vδ2 TCRs. Circulating Vγ9Vδ2 T cells dramatically expand during certain infections, such as tuberculosis [119]. An equivalent subset of human Vγ9Vδ2 cells is absent in mice.
Vγ9Vδ2 T cells recognize low-molecular–weight nonpeptide phosphorylated antigens, called phosphoantigens [104, 119]. Phosphoantigens include (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate (HMBPP), which is an intermediate metabolite in the 2-C-methyl-D-erythritol 4-phosphate (MEP) pathway of microbial isoprenoid biosynthesis, and isopentenyl pyrophosphate (IPP), which is an intermediate metabolite in the self mevalonate pathway, but IPP is only active at high concentrations. Infection with most bacteria and protozoa using the MEP pathway (staphylococci and streptococci do not use the MEP pathway) induces rapid expansion of Vγ9Vδ2 T cells through recognition of HMBPP. Vγ9Vδ2 T cells can also recognize high levels of IPP accumulated in stressed cells or tumor cells. Vγ9Vδ2 T cells are also indirectly activated by aminobisphosphonates and alkylamines that inhibit farnesyl pyrophosphate synthase, leading to accumulation of IPP. Therefore, Vγ9Vδ2 T cells can contribute to immunity against both pathogens (by recognizing microbial phosphoantigens) and tumors (by recognizing overproduced endogenous phosphoantigens). The mechanism by which phosphoantigens activate Vγ9Vδ2 T cells remains unclear, but butyrophilin 3A1 has been identified as a candidate antigen presenting molecule for phosphoantigen-reactive Vγ9Vδ2 T cells [120, 121].
Like conventional αβ T cells, Vγ9Vδ2 T cells can be subdivided into four subsets based on the expression of CD27 and CD45RA: CD27+ CD45RA+ naive, CD27+ CD45RA−central memory (TCM), CD27−CD45RA−effector memory (TEM), and terminally differentiated cytotoxic CD27−CD45RA+ effector memory (TEMRA) cells [122, 123]. Vγ9Vδ2 T cells expand significantly during the perinatal period [124] and most of the Vγ9Vδ2 T cells acquire a memory phenotype by 1 year of life [123]. In contrast, about 30–40% of Vδ1 T cells remain naive in young adults [123].
Vγ9Vδ2 T cells secrete IFN-γ upon TCR stimulation, but can be polarized to Th1-, Th2-, Th17-, TFH-, or Treg-like cells [125, 126]. Vγ9Vδ2 T cells can produce connective tissue growth factor (CTGF) [127] and FGF9 [128], and may be involved in wound healing and pathological fibrosis.
IL-17-producing Vγ9Vδ2 T cells can be induced or expanded in vitro, especially from neonates [53, 129–131]. IL-17-producing Vγ9Vδ2 T cells are CD161(NK1.1)+ CCR6+ and show the CD27−CD45RA+ TEMRA phenotype [53, 131, 132]. They expand during bacterial infection [131]. A subset of circulating Vγ9Vδ2 T cells that express CLA and CCR6 can be rapidly recruited into perturbed skin [133]. These skin-homing Vγ9Vδ2 T cells produce proinflammatory cytokines including IL-17A, chemokines, and IGF1, and are implicated in the development of psoriasis [133]. Indeed, increased numbers of γδ T cells, which produce IL-17A upon IL-23 stimulation, are present in the skin lesions of psoriasis patients [4, 134].
Vγ9Vδ2 T cells are potent cytotoxic T cells and recognize infected cells or tumor cells through the TCR and/or NKG2D [135–138]. CD56 expression correlates with cytotoxicity in Vγ9Vδ2 T cells [139, 140]. Vγ9Vδ2 T cells have been tested for cellular immunotherapies in clinical trials for various cancers [141].
References
1.
Witherden DA, Havran WL (2011) Molecular aspects of epithelial gammadelta T cell regulation. Trends Immunol 32:265–271PubMedPubMedCentral
2.
Macleod AS, Havran WL (2011) Functions of skin-resident gammadelta T cells. Cell Mol Life Sci 68:2399–2408PubMedPubMedCentral
3.
Sumaria N, Roediger B, Ng LG, Qin J, Pinto R, Cavanagh LL, Shklovskaya E, Fazekas de St Groth B, Triccas JA, Weninger W (2011) Cutaneous immunosurveillance by self-renewing dermal gammadelta T cells. J Exp Med 208:505–518PubMedPubMedCentral
4.
Cai Y, Shen X, Ding C, Qi C, Li K, Li X, Jala VR, Zhang HG, Wang T, Zheng J, Yan J (2011) Pivotal role of dermal IL-17-producing gammadelta T cells in skin inflammation. Immunity 35:596–610PubMedPubMedCentral
5.
Dupuy P, Heslan M, Fraitag S, Hercend T, Dubertret L, Bagot M (1990) T-cell receptor-gamma/delta bearing lymphocytes in normal and inflammatory human skin. J Invest Dermatol 94:764–768PubMed
6.
Bos JD, Teunissen MB, Cairo I, Krieg SR, Kapsenberg ML, Das PK, Borst J (1990) T-cell receptor gamma delta bearing cells in normal human skin. J Invest Dermatol 94:37–42PubMed
7.
Foster CA, Yokozeki H, Rappersberger K, Koning F, Volc-Platzer B, Rieger A, Coligan JE, Wolff K, Stingl G (1990) Human epidermal T cells predominantly belong to the lineage expressing alpha/beta T cell receptor. J Exp Med 171:997–1013PubMed
8.
Toulon A, Breton L, Taylor KR, Tenenhaus M, Bhavsar D, Lanigan C, Rudolph R, Jameson J, Havran WL (2009) A role for human skin-resident T cells in wound healing. J Exp Med 206:743–750PubMedPubMedCentral
9.
Ebert LM, Meuter S, Moser B (2006) Homing and function of human skin gammadelta T cells and NK cells: relevance for tumor surveillance. J Immunol 176:4331–4336PubMed
10.
Hayday AC (2009) Gammadelta T cells and the lymphoid stress-surveillance response. Immunity 31:184–196PubMed
11.
Bonneville M, O’Brien RL, Born WK (2010) Gammadelta T cell effector functions: a blend of innate programming and acquired plasticity. Nat Rev Immunol 10:467–478PubMed
12.
Garman RD, Doherty PJ, Raulet DH (1986) Diversity, rearrangement, and expression of murine T cell gamma genes. Cell 45:733–742PubMed
13.
Allison JP, Havran WL (1991) The immunobiology of T cells with invariant gamma delta antigen receptors. Annu Rev Immunol 9:679–705PubMed
14.
Haas JD, Ravens S, Duber S, Sandrock I, Oberdorfer L, Kashani E, Chennupati V, Fohse L, Naumann R, Weiss S, Krueger A, Forster R, Prinz I (2012) Development of interleukin-17-producing gammadelta T cells is restricted to a functional embryonic wave. Immunity 37:48–59PubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree